HPLC or High-performance liquid chromatography or a Highly Improved form of Column Chromatography is an analytical flexible technique in the field of analytical chemistry used for the separation of components of an organic mixture of compounds when such compounds are nonvolatile, thermally unstable, and have relatively highly molecular weights. It can be possible to analyze a sample over a vast concentration range and molecular weights by this technique.
This technique was discovered by M. S. T Swett in around 1900 to study leaf pigments. It is a special type of column chromatography that pumps an analyte or sample mixtures in a solvent (mobile phase) at high pressure through a column with chromatographic packing material (stationary phase). The sample mixture is generally carried by a moving carrier gas stream of nitrogen or helium.
Sample retention time must be varied depending on the interaction between the stationary phase and mobile phase. When the sample passes through the column it interacts between the two phases at different rates, primarily because of different polarities in the analytes. Analytes that have the lower amount of interaction with the stationary phase or the most amount of interaction with the mobile phase will elute the column faster. The amount of retardation basically depends on the properties of the analyte and the composition of both the mobile and stationary phases. The most common solvents used in HPLC systems are methanol and acetonitrile.
Principle of High-Performance Liquid Chromatography (HPLC)
The purification of various samples of this technique takes place in a separation column between a stationary and a mobile phase. The stationary phase is made of granular material with very small porous particles in a separation column. The mobile phase is a solvent or solvent mixture that is forced through the column by high pressure delivered through a pump.
The molecules can be separated while passing through the stationary phase, depending on the chemical properties and structure of the analyte. The duration time a sample spends “on-HPLC column” is identified by the intermolecular interactions between its molecules and the packing material. As a result, the various constituents of a sample get eluted at different times, and hence the separation can be possible. The separated constituents are then identified by a detector that can measure their amount in that mixture. The output from the detector is known as a “liquid chromatogram.”
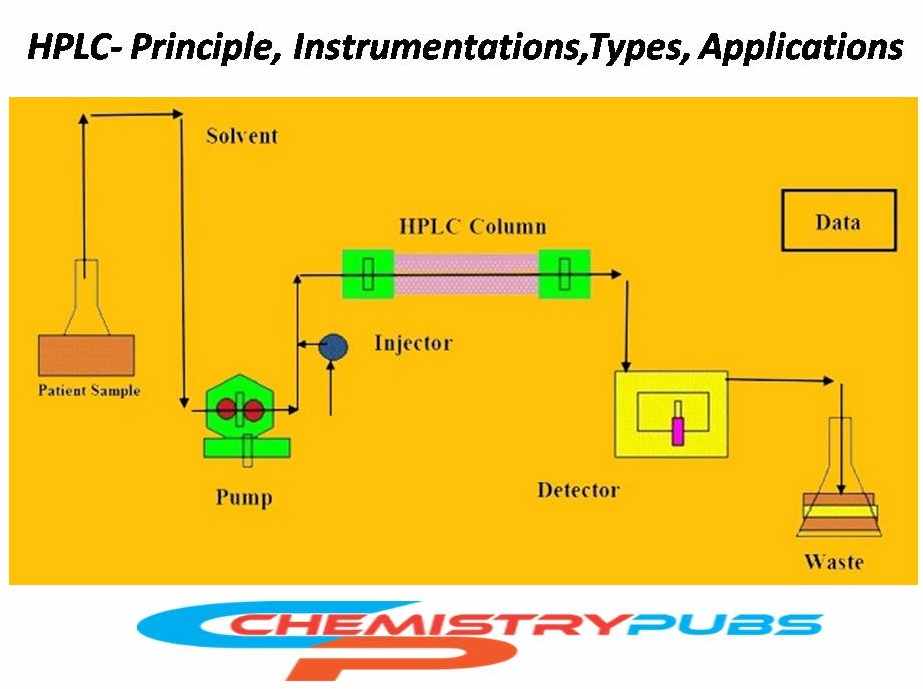
Instrumentation of High-Performance Liquid Chromatography (HPLC)
Solvent reservoir: This portion is also known as a mobile phase reservoir. It should not use highly viscous solvents as it takes much more time to travel through the column. A higher amount of pressure is required for the viscous solvent. Different types of solvents are used in HPLC systems, such as aqueous solvents (water) and organic solvents (, acetonitrile, methanol, and propanol). Acetic acid, formic acid, and trifluoroacetic acid can be used for the improvement of chromatographic peak shape.
Pump: The HPLC pump can deliver a constant mobile phase composition (isocratic) or a rising mobile phase composition (gradient) during the identification, and quantification of various components from the mixture.
Injector: The injector is generally used to push the liquid sample into the stream of the mobile phase. The range of the tested sample should be 5 to 20 microliters (L).
HPLC Column: It is the main part of the chromatograph. The length of the HPLC column generally varies from 5 cm to 30 cm, and its diameter ranges from 2 mm to 50 mm. Generally, stainless steel is used for making the HPLC tube, and silica or alumina particle is used as packing materials. The mobile phase is delivered from the solvent reservoir and forced through the system’s column and detector by a pump.
Detector: The detector identifies individual molecules leaving the column and delivers an output to a computer or recorder for producing a liquid chromatogram.
Computer: It receives the signal from the detector and analyzes the retention time. Then we can analyze the constituents qualitatively and quantitatively.
Types of HPLC
There exist many types of this technique depending upon the system of phase:
1. Normal Phase HPLC
This type of technique known as absorption chromatography or normal-phase chromatograph separates the analytes based on their polarity. This chromatography has a non-polar mobile phase and a polar stationary phase. Silica is used in this chromatography as the stationary phase and hexane, methylene chloride, chloroform, and diethyl ether are used as the mobile phase. This technique is used for the separation of geometric isomers, cis-trans isomers, class separations, water-sensitive compounds, and chiral compounds.
2. Reverse Phase HPLC
In this type of technique, the mobile phase is moderately polar and the stationary phase is nonpolar. It follows the hydrophobic interactions principle. The more nonpolar material has a longer retention time. This HPLC system is suitable for the separation of non-polar, polar, ionizable, and ionic molecules from the mixture.
3. Size-exclusion HPLC
This chromatography is also known as gel permeation chromatography or gel filtration chromatography.
The column of this chromatography is filled with precisely controlled pore sizes of material, and the particles are separated according to their molecular size. The smaller size molecules have a longer retention time than the larger molecules. This technique is helpful in determining the tertiary and quaternary structure of proteins and amino acids. It is also helpful for the identification of the molecular weight of polysaccharides.
4. Ion-Exchange HPLC
In Ion-exchange-type chromatography, retention time is based on the interactions between solute ions and charged sites bound to the stationary phase. This chromatographic technique is generally used for purifying water.
5. Bio-affinity HPLC
In this technique, separation is completed by the reversible interaction of proteins with ligands.
Applications of High-Performance Liquid Chromatography
The system of this technique has been developed for use in almost all areas of chemistry, biochemistry, and pharmacy for the resolution, identification, and quantification of a compound. It also helps in chemical separation and purification of the components from the mixture. The important applications of this system are given below:
To check the drug stability.
To the analysis of Tablet dissolution study of pharmaceutical dosages form.
Analysis and identification of synthetic polymers
Analysis of various harmful pollutants in environmental analytics
Analysis of various drugs in biological matrices
Isolation and purification of valuable products from natural sources
Industrial Product purification purposes
Quality control of various industrial products and fine chemicals
Identification, separation, and purification of biopolymers such as enzymes or nucleic acids
Water purification from various wastage sources
Advantages and Limitations of High-Performance Liquid Chromatography
There are many advantages of using this technique is the following:
It has good speed for the resolution, identification, and quantification of a compound.
This system has the greatest efficiency.
The accuracy of this technique is high.
The versatility of identifying and quantifying chemical components is higher than the others.
It can test the various contaminants and other impurities in the sample mixtures.
It develops better products.
Limitations of High-Performance Liquid Chromatography
There are some limitations to using HPLC is the following.
The resolution, identification, and quantification of various components from the mixture by the HPLC system require a large number of expensive organics.
HPLC has a system complexity than the other chromatographic techniques.
It has lower sensitivity for certain compounds, and some cannot even be detected as they are irreversibly adsorbed.
References:
Corradini, D., Eksteen, E., Eksteen, R., Schoenmakers, P., & Miller, N. (1998). Handbook of HPLC. CRC Press.

