Bee- Lambert law is one of the most important topics in the field of analytical chemistry which relates the attenuation of light and the properties of the substance during traveling through a medium. The derivation of this law helps to understand the relationship between the intensity of visible UV radiation and the amount of substance present. This law has versatile applications for testing medicines, organic chemistry, and tests with quantification.
Beer-Lambert Law Statement
Beer-Lambert law is the combination of Beer and Lambert law which are given below:
Lambert’s Law
The intensity of the transmitted monochromatic radiation is gradually decreased when the thickness of the absorbing medium is increased.
The mathematical expression of Lambert’s law is given below:
-(dI0/dC)=K’I0………………………..(1)
The negative sign of the above equation expresses the intensity of the transmitted incident light radiations.
The above equation (1) can be rewritten as follows:
It=I0 10-K’b……………………………(2)
Here, It = Intensity of transmitted light radiation
k’ = Proportionality constant
We can also write the equation (1) in the following way:
A=log (I0/It)∞ b
The above expression says that the absorbance of incident light radiation in a homogenous medium is directly proportional to the thickness of the medium.
The simple expression of the above equitation can be written as follows:
So, A = εb
Where, ε= Molar Absorption Coefficient
Beer’s Law
The rate of decrease of transmitted monochromatic radiation through a ‘transparent medium’ like a solution with the increase in the concentration of the medium is linearly proportional to the intensity of the incident light.
It can be said in an easy way that the concentration of the solution and absorbance are directly proportional to each other.
Mathematically this law can be expressed as follows:
−(dI0/dC)= KI0 ………………..(3)
Here, Io= Intensity of incident radiation
We can rewrite this equation as:
It=I0 10-K’’C……………………(4)
Where, It= Intensity of transmitted radiation
k’’ = Proportionality constant
Another form of writing equation (3) is given below:
A=log (I0/It)∞ b
The above expression means that the absorbance of incident light radiation in a homogenous medium is directly proportional to the concentration of the sample.
Beer-Lambert Law Derivation
To derive the Beer-Lambert Law equation, we can combine the equation (2) & (4), and take the log of these, and then we get the following equation:
log (I0/It)=K’K’’bc…………………………….(5)
We can express the above (5) no equation as follows:
A= εbc………………………………………….(6)
The above equation (6) is the final form Beer-Lambert Law Formula.
Where,
A = Absorption of incident light radiation
ε = Molar Absorption Coefficient or Molar Extinction Coefficient Molar Absorptivity in m-1cm-1 = k’ x k’’
b = Thickness of the medium express in cm
c = Molar concentration express in M (molarity)
So we can say that the Beer-Lambert Law is as follows:
“When a beam of monochromatic light is passed through a homogeneous medium of an absorbing substance then the rate of decrease in intensity of transmitted light radiation with the thickness of absorbing medium is directly proportional to the intensity of radiation as well as to the concentration of the solution.”

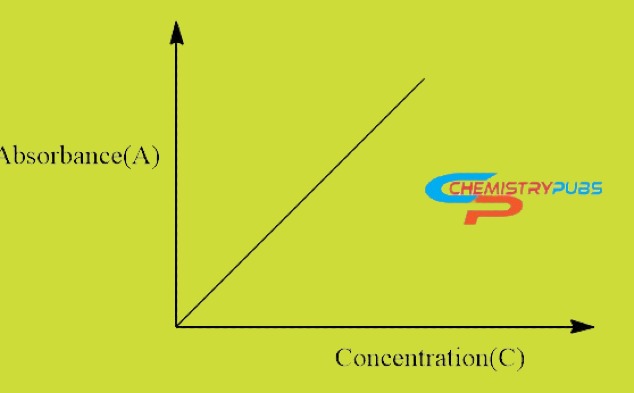
Beer-Lambert law graph
From Beer Lambert’s law, we have
A= εbc
Where,
A = Absorption of incident light radiation
ε = Molar Absorption Coefficient or Molar Extinction Coefficient Molar Absorptivity in m-1cm-1 = k’ x k’’
b = Thickness of the medium express in cm
c = Molar concentration express in M (molarity)
The graphical representation of Beer-Lambert Law is used to determine the value of the Molar Extinction Coefficient (ε). This Molar Extinction Coefficient value is determined easily by plotting the values of absorbance (on the y-axis) against various values of concentrations (on the x -axis) and determining slope εb which is obtained.
Beer-Lambert law Limitations
The Beer-Lambert law Limitations are given below:
This law is only valid for monochromatic light radiation sources.
This law can be used only in a low concentration range where interactions between molecules are not considered.
This law is not applicable when radiations of very high intensities are used.
Beer-Lambert law Deviation
The Beer-Lambert law Deviations are given as:
The Beer-Lambert law Deviation mainly occurs when the light of a single wavelength is not used
The phenomenon of association, dissociation, or ionization of solutes causes deviation
This law falls on deviation when the polymerization of various solutes during the measurements occurs
Beer-Lambert Law Applications
There are various applications of Beer-Lambert Law which are given below:
The harmful dust particles in the atmosphere can be calculated using the formula.
This law is very essential to analyze various pharmaceutical products.
Electromagnetic spectroscopy is constructed by following this law.
The molar absorbance of bilirubin in blood plasma samples can be detected easily by following this law.
The qualitative and quantitative analysis of biological and domestic material samples containing inorganic and organic substances can be conducted by following the Beer-Lambert law.
References
1.Swinehart, D. F. (1962). The beer-lambert law. Journal of chemical education, 39(7), 333.
2. Calloway, D. (1997). Beer-lambert law. Journal of Chemical Education, 74(7), 744.

