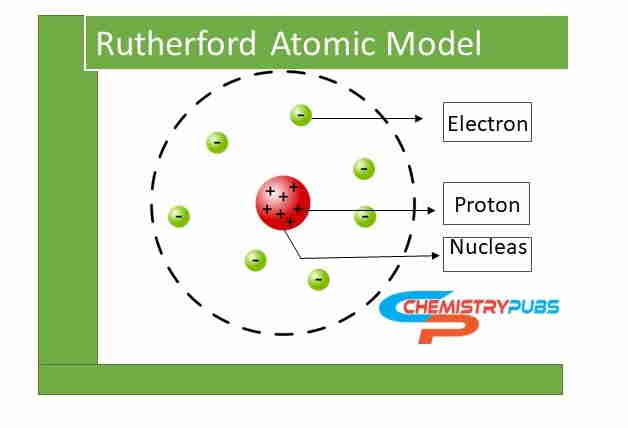
Rutherford Atomic Model or planetary model of the atom relates the atomic structure of elements which was proposed by Ernest Rutherford in 1911. This model described that an atom in a molecule has a small, dense, and positively charged core called a nucleus.
All the mass of the atom is generally concentrated in the nucleus and the negatively charged electrons move around the nucleus, called electrons. The revolving electrons move around the nucleus at some distance like planets revolving around the Sun. This model was invented by Ernest Rutherford after conducting an experiment to investigate the composition of gold foil by using alpha particles.
What is the Rutherford Atomic Model?
This model describes that the positively charged particles and most of the mass of an atom are concentrated in a small volume area called the nucleus. The negatively charged particles called electrons revolve around the nucleus like the velocity of light. The moving circle of the electron around the nucleus is called the orbit. This model gives the idea about the nucleus and electron to know the structure of the atom.
Diagram of Rutherford Atomic Model
The diagram of the Rutherford Atomic Model is shown below:
History of Rutherford Atomic Model
A Greek philosopher Democritus in 400 BCE proposed the idea about an atom. But there were various drawbacks. The modern concept of the structure of the atom was proposed by John Dalton in 1803. He describes some postulates about the atom with scientific explanations. He proposed that an atom in a molecule is indivisible. British physicist J.J. Thomson in 1897 discovered and could be a separate negatively charged particle electron that is found in an atom.
After some years, a new atomic model was given by Ernest Rutherford in 1911. Rutherford conducted an experiment in which a thin gold sheet was bombarded by alpha particles. He invented that every atom contains positively charged particles at the center of the atom’s known nucleus. All about the mass of the atom concentrated in this area.
Rutherford Gold Foil Experiment
Rutherford selected a gold foil which had an extremely thin layer. He assumed that the gold foil thickness contained about 1000 atoms. Rapidly passing α-particles were produced from spontaneously radioactive decay of polonium isotopes which contained a mass of about 4 amu. Then the α-particles were directed towards the gold foil. Some α-particles were deflected by heavier protons.
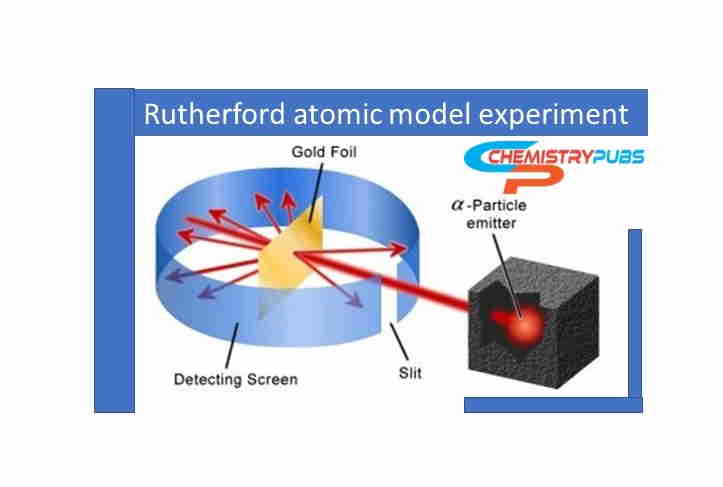
Most of the α-particles transmitted through the gold foil without any deflection. He invented that the positively charged particle in an atom is concentrated in a relatively small volume which is not dispersed mostly. The positively charged particles protons occupy a small volume area of an atom’s overall volume. Most of the area contained by an atom is empty.
After conducting the above experiment, Rutherford concluded the following matter:
He observed that about 1 in 8000 alpha particles changed their direction quite smoothly. The angle of this deflection was more than 90°.
The majority of the space containing an atom is vacant because most of the α-particles transmitted through the gold sheet without any deflection.
A little space contains positively charged which is responsible for diverting a few α-particles in almost 180 degrees angle of deflection during transmission.
He suggested that the positively charged particles in an atom remain in a small area.
What is an alpha particle?
It has a good amount of energy and contains a charge of +2. Actually, it is the nucleus of a helium atom.
Why did Rutherford use gold foil to conduct this experiment?
Gold foil contains a thin layer that is much heavier than protons. It was very important for conducting Rutherford’s research.
Postulates of Rutherford Atomic Model
The major postulates of this atomic model after conducting the gold foil experiment are given below:
The majority of the mass of an atom is concentrated in an extremely small volume area. This area of an atom is called the nucleus.
The negatively charged electrons revolve around the nucleus in a fixed circular path of an atom at a very high speed. The circulation path of the electron around the nucleus is known as orbit.
The negatively charged electrons around the nucleus and positively charged protons in the nucleus are held together by a strong electrostatic force of attraction. As a result, an atom either has no net charge or is electrically neutral for this reason.
Limitations of the Rutherford Atomic Model
There exist some drawbacks or limitations of this atomic model which are given below:
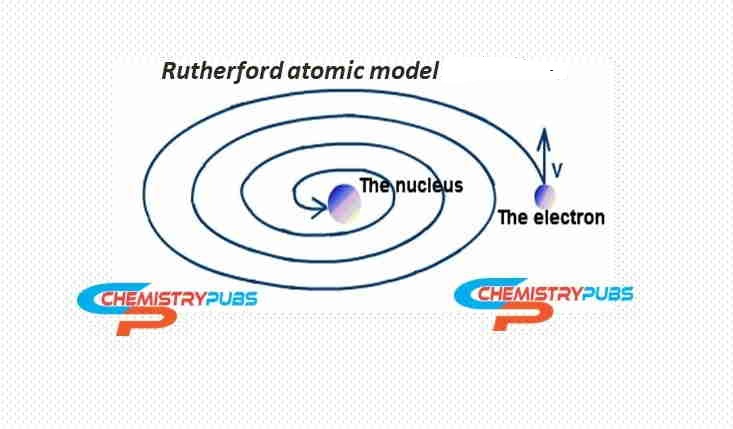
This model predicts that the electrons in orbit will revolve around the positively charged nucleus, which is not anticipated to be stable.
The charged particles around the nucleus when moving with a rapid motion, will lose energy continually and eventually. Then the charged particles collapse into the nucleus which makes these particles to be unstable. So, this atomic model fails to explain the stability of atoms.
This model is unable to solve the problem of atomic mass issues.
This model cannot explain the matter of the arrangement of electrons in the atom
It is generally found to a discontinuous in the form of characteristic lines of definite wavelengths in hydrogen atoms. This model cannot explain the existence of certain definite lines in the hydrogen spectrum.
Advantages of Rutherford atomic model
We get the information about the nucleus from the Rutherford experiment.
We know the movement of electrons around the nucleus.
It can be known that most of the atom spaces are hollow.
It can be known the reason behind the atomic neutral characteristic when no electrons are transferred.
Conclusion of Rutherford atomic model
There are some points that can be concluded for Rutherford’s atomic model. All the positively charged and the entire mass number of the atom are concentrated in a small central core area known as the nucleus. The radius of the atomic nucleus is extremely small which may be 10−13m. The radius of the atom is approximately 10−8 m. Most of the area containing an atom is vacant. The inside nucleus and the outside electrons make the atom ultimately neutral.
Frequently Asked Questions (FAQ’s)
What is the Rutherford atomic model?
The model related to the structure of elements discovered by Rutherford is called Rutherford atomic model. He suggested that a positively charged particle lies inside the atom where the mass of an atom is concentrated. The negatively charged electrons revolve around the nucleus with high velocity. There is an electrostatic force of attraction between the nucleus and the electron.
What are the limitations of Rutherford’s atomic model?
This model is unable to explain the electronic arrangement of electrons in an atom. It cannot be described about the spectral matter of atoms by this model. The paths of the revolving electron cannot be explained with the help of this model.
What are the postulates of Rutherford’s atomic model?
This model gives the idea that the nucleus contains positively charged protons. It can understand the structure of atoms by this model. The information about the neutral characteristics of atoms can be known by the postulates of Rutherford’s atomic model.