Isotopes are atoms of the same element having the same atomic numbers (Z) but different numbers of neutrons giving them different atomic masses. The position of isotopes in the periodic table is the same but with different atomic masses. As a result, the different numbers of neutrons are available in the nucleus of that atom. Isotopic atoms have almost similar chemical properties but different physical properties for the presence of different atomic masses.
Atoms contain a cloud of electrons surrounding a nucleus and are comprised of protons and neutrons. The number of protons in an atom is a unique characteristic. For example, a krypton nucleus always has 36 protons, and a rubidium nucleus always has 37. Atoms are electrically neutral for general purposes because they contain an equal number of electrons surrounding the nucleus.
The number of protons or atomic number is the major characteristic of an atom. The chemical properties of an atom depend on this number and it is denoted by the symbol Z. The atomic mass number represents the total number of nucleons (protons and neutrons) in an atom which is denoted by the symbol A. The neutron numbers in an atom are represented by the symbol N. So, we can write the total atomic mass of an atom as A = N + Z.
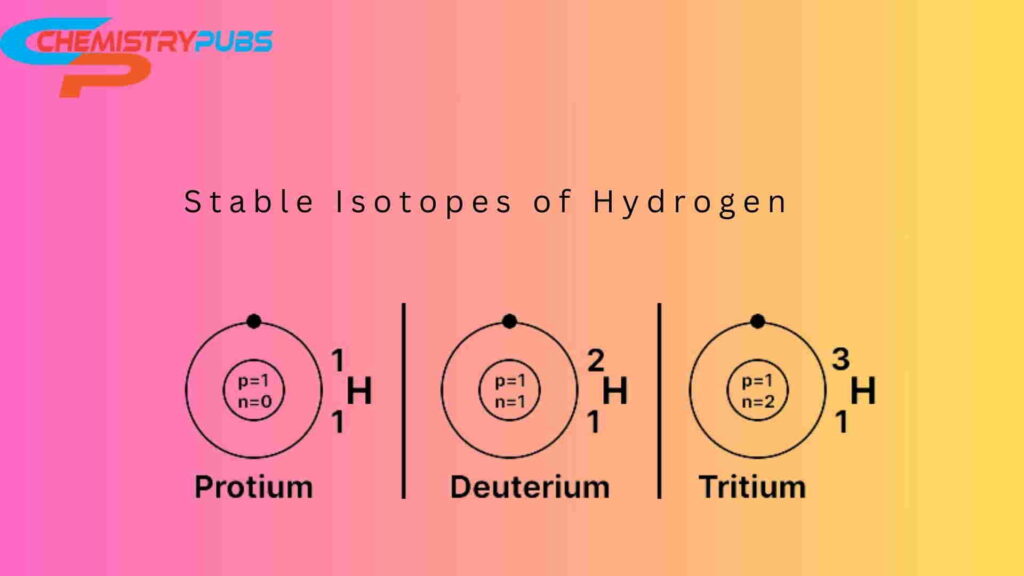
Hydrogen has three natural type isotopes namely Protium (1H), Deuterium (2H), and Tritium (3H). Carbon-12 is a stable isotope, whereas Carbon-14 is an unstable (radioactive) isotope. Oxygen-16, Oxygen-17, and Oxygen-18 isotopes are unstable and synthesized in labs.
There are mainly two types of isotopes namely stable and unstable isotopes in our planet. Stable-type isotopes will decay over time and can be turned into another element or another isotope. Stable isotopes are found in nature. An unstable isotope emits radiation and undergoes radioactive decay. The alpha, beta, and gamma rays are generally emitted by unstable isotopes. It is an unstable combination of protons and neutrons in an unstable isotope or radioactive isotope. Unstable isotopes are classified into long-lived, cosmogenic, anthropogenic, and radiogenic categories based on their creation process.
Stable isotopes are used very much to determine the various characteristics of geological materials, such as their age and where they came from. For example, the Carbon 14 isotope is used to identify the presence of carbon in the fossils and help in calculating the age of fossils. Uranium-235 isotope is widely used as a fossil fuel in nuclear reactors. Unstable or radioactive isotopes are used in the field of medicine for identifying and curing various diseases such as cancer, tumors, goiter, etc.
Isotopes Definition
It is defined as the variations of the same element containing the same number of protons and electrons with a different number of nucleons in their respective nuclei.
We know that atomic mass or mass number is the combination of protons and neutrons. The atomic number of an element gives the idea about the total number of protons. Sometimes, the neutron numbers are found to be changed but the number of protons always remains the same in certain elements. These elements are termed as isotopes.
Isotope Examples
Carbon-12 is a naturally occurring isotope and carbon-14 is an unstable radioactive isotope. Uranium-235 and uranium-238 are found in the Earth’s crust.Protium(1H1), Deuterium (1H2), and Tritium(1H3) are found in nature. Oxygen-16, Oxygen-17, and Oxygen-18 are stable and found in nature.
Isotope Types
There are mainly two types of isotopes namely stable and unstable (radioactive) isotopes based on their stability which discussions are given below:
Stable Isotope
It has long half-lives (in the order of hundreds of millions of years). It contains stable nuclides and can not degrade easily. For example: carbon-12, carbon-13, oxygen-16, oxygen-17, and oxygen-18.
Unstable Isotope
It has short half-lives and degrades quickly after emitting radioactive waves. For example: Tritium (Hydrogen-3), Carbon-14, chlorine-36, uranium-235, and uranium-238.
Isotope Formation and Types of Radiation
Isotopic elements can be formed spontaneously in nature after the emission of energy in the form of alpha particles, beta particles, neutrons, and photons. Radioactive new or existing isotopic elements can be produced artificially in a nuclear reactor by bombarding a stable nucleus with charged particles via accelerators or neutrons. The conversion process of one element to another element is called “transmutation.”
Chemical properties of isotope
Isotopic atoms contain different-sized nuclei.
They show various reactions on the basis of different temperatures and pressures.
Each unstable isotope has a half-life which ranges from a fraction of a second to billions of years long.
Each unstable isotope has the property to change spontaneously over time after radioactive decay.
A parent isotope can transform into a “daughter” isotope after radioactive decay.
Physical properties of the isotope
They change their density when change their mass.
Fractional distillation and diffusion processes are used to separate the isotopic atoms from each other.
The mass of each isotopic atom varies from one another.
History of isotopes
The stable type isotopes were first introduced by J. J. Thomson in 1912 during an experiment about knowing the composition of canal rays (positive ions). J. J. Thomson conducted an experiment to know the deflection of neon ions through parallel magnetic and electric fields. He placed a photographic plate in the path of neon ions and computed their mass-to-charge ratio using a method known as Thomson’s parabola method.
A glowing patch was created on the plate by each ion stream at the point it struck the photographic plate. Then it was possible to introduce the two species of nuclei with different mass-to-charge ratios.
The existence of radioactive isotope was first discovered by the Chemist Frederick Soddy in 1913 after knowing the matter of radioactive decay chains produced an element. He introduced the effect of the alpha decay emission produced by an element two places to the left in the periodic table, whereas beta decay emission occurred by an element one place to the right.
Frederick Soddy noted that the alpha particle emission followed by beta particle emission could be formed of an element that was chemically identical to the initial element but with a mass four units lighter and contained different types of radioactive characteristics.
Uses of Isotopes
Carbon-14, an isotope of Carbon is used to calculate the age of the fossils.
Uranium-235 isotope is used as a fuel in nuclear reactors to produce electricity.
Arsenic-74 isotope is used in the field of medicine for determining the presence of a tumor.
Sodium-24 isotope is used in the field of medicine for the detection of blood clots.
Cobalt (cobalt-60) isotope is used in the field of medicine for the treatment of cancer.
Iodine (Iodine-131) isotope is used in the field of medicine for the treatment of goiter.
Cobalt-60 or caesium-137 isotopes are used in the food industry to emit radiation that can be used to kill microorganisms on a variety of foodstuffs, thereby extending their shelf life like mushrooms, sprouts, and berries.
Technetium-99 isotope is used to examine the function of the thyroid.
Americum-241 isotope is used in homes and industrial sectors to detect smoke.
Cadmium-109 isotope is used to analyze the various metal alloys for stock control and scrap sorting.
Calcium-47 isotope is used to study the cellular functions and bone formation in mammals.
Californium-252 isotope is used to detect the explosives in luggage and to determine the moisture content of soil in the road and building industries.
Caesium-137 isotope is used in the field of medicine to treat cancerous tumors. It is also used to measure and control liquid flow in oil pipelines. The fulfillment of food, drugs, and other products in packages can be identified by applying this isotope.
Cobalt-57 isotope is used as a good tracing element to diagnose pernicious anemia.
Copper-67 isotope is used to destroy the tumor cell.
Curium-244 isotope is used for analyzing the excavated material from pits and slurries from drilling operations.
Gallium-67 isotope is used in the medical field to identify various diseases.
Strontium-90 isotope is used in various institutions for the survey of meters.
Sulphur-35 isotope is used in biotechnology and genetic engineering laboratories for various research purposes.
Technetium-99 isotope is used in medical fields to know the functions of bone, liver, spleen, brain, and kidney imaging, as well as blood flow studies.
Thallium-201 isotope is used in nuclear medicine for nuclear cardiology and tumor detection.
Thorium-229 isotope is used to increase the strength of fluorescent lights.
Iodine-123 isotope is used in the medical field to diagnose Thyroid disorders and various problems in brain functions.
Iodine-125 isotope is used in the medical field for clinical tests and to diagnose thyroid disorders.
Nickel-63 isotope is used to detect the explosive. It is also used in electronic devices, and electron capture detectors in gas chromatographs.
Plutonium-238 isotope is used for the production of photographic film and other materials to increase stability.
Polonium-210 isotope is used to identify the thickness of thermoplastics, metallic sheets, paper, rubber, and various textile products.
Radium-226 isotope is used to enhance the strength of lightning rods
Sodium-24 isotope is used in the industrial sectors to detect leakages in pipelines and oil well studies.
Strontium-85 isotope is used to know the bone formation and metabolism characteristics in the human body.
Thorium-230 isotope is used for making colored glazes and glassware.
Xenon-133 isotope is used in nuclear medicine in the field of medicine to study lung ventilation and blood flow.
Frequently Asked Questions (FAQs)
What is an isotope?
It is defined as the variants of a specific element having the same atomic numbers but differing in the number of neutrons in the atom.
What are the types of isotopes?
There are mainly two types of isotopes namely stable isotope and radioactive unstable isotope.
What are the examples of isotopes?
Carbon-12, Carbon-14, Uranium-235, Uranium-238, Oxygen-16, Oxygen-17, Oxygen-18,Protium (1H), Deuterium (2H), and Tritium (3H)
Who invented the isotope?
A stable isotope was invented by J. J. Thomson in 1912, whereas a radioactive unstable isotope was invented by Frederick Soddy in 1913.
What are the properties of isotopes?
The atomic masses of each isotopic atom are different from one another. Fractional distillation and diffusion are used to separate the isotopic atoms from each other.
What are the uses of isotopes?
Isotopes are widely used in the field of medicine to identify and treat various types of diseases. Isotopic materials are used to manufacture various sensors. Isotopic elements are used in food, chemical, electrical, nuclear, and electronic industries to prepare different types of materials. They are used in the agricultural sectors to identify the characteristics of soil.
What is radioisotope?
It is defined as the variants of a specific element produced artificially in nuclear reactors having an unstable combination of protons and neutrons. They have the ability to emit alpha, beta, and gamma radiation and turn into different elements after a long time.
What are the isotopes of chlorine?
It has 25 isotopes, ranging from 28Cl to 52Cl. Clorine-35 and Chlorine-37 are mostly used.
What are the isotopes of hydrogen?
Protium (1H), Deuterium (2H), and Tritium (3H) are natural isotopes of hydrogen.
What are the isotopes of oxygen?
They are Oxygen-13, Oxygen-14, Oxygen-15, Oxygen-16, Oxygen-17, Oxygen-18, Oxygen-28.
What are the isotopes of Uranium?
They are Uranium-230, Uranium-234, Uranium-235, Uranium-236, Uranium-237, Uranium-238, and Uranium-241.
What are the isotopes of carbon?
Carbon-12, Carbon-13, and Carbon-14 are stable types, whereas Carbon-16, Carbon-17, Carbon-18 are unstable types.

