Quantum dots (QDs) are man-made very tiny semiconducting materials, such as graphene, selenite, or metal sulfides containing diameters at the nanometer level. The diameter of the quantum dot ranges between 2 to 10 nanometers. Their smaller size helps to show various attractive optical and electronic properties different from those of bulk matter.
The Nobel Prize in Chemistry 2023 was awarded to Moungi G. Bawendi, Louis E. Brus, and Alexei I. Ekimov for discovering and developing Quantum dots (QDs) in the field of nanochemistry.

Quantum dots (QDs) were first synthesized in 1980, though the theory of QDs was introduced in 1970. They have the most attractive optical and electronic properties because they can produce distinctive colors determined by the size of the particles. They have the ability to transport electrons from their valence shell and emit light of various colors after being exposed to UV light.
Quantum dots (QDs) nanoparticles create various fields of applications including use in polymeric and other composites, fluorescent biological labeling, LEDs, solar cells, lighting, photovoltaic devices Bio-imaging, and so on.
What are Quantum Dots?
Quantum dots are manmade nanocrystals having semiconducting properties. They show excitation spectra, narrow emission spectra, tunable emission peaks, and long fluorescence lifetimes. They have the ability to conjugate with proteins and create excellent probes for bioimaging applications and others.
Their size is not more than 10 nanometers. So we can understand that their size is very small and makes them different to show various attractive optical and electronic properties. They emit mostly shorter wavelengths generating colors such as violet, blue, or green when they have a radius of 2 to 4 nm. They also generate longer wavelengths generating colors like yellow, orange, or red when they have a radius of 5 to 10 nm.
Researchers are attracted by QDs for their tiny size and properties. They create a lot range of applications including composite, solar cells, LEDs, Photovoltaic devices, diode lasers, transistors, etc. for having their fascinating size.
What is Quantum dots made of?
QDS are artificial semiconductor nanocrystals. They are formed by a few hundred to a few thousand atoms of cadmium sulfide, cadmium selenide, cadmium telluride, etc. They possess attractive optical properties due to exciton confinement, based on the size of the QDs nanocrystals. QDs were first reported in 1983 by Rossetti, Nakahara, & Brus.
Quantum dots examples
Quantum dots are popular nowadays. They are found in LED displays to increase the color and brightness of screens. QDs are found in solar cells to develop their efficiency because they have attractive light absorption properties and are useful for capturing direct sunlight more effectively.
Quantum dots are found in tools of cell biology and medical diagnostics because they have various attractive optical properties, including bright and stable fluorescence. QDs are found in semiconductor lasers because of have tunable emission wavelengths and threshold currents that make the semiconductor lasers more valuable for communication and data storage.
Quantum dots are found in television backlighting because they develop color reproduction. They are very much found in photodetectors because they have the ability to convert photons into electrical signals within a second. They are found in sensors, lasers, and photovoltaic devices. QDs nanocrystals are also found in various industries, including anti-counterfeiting measures, security printing, and tracking and tracing applications.
The above examples focus on the versatility and growing importance in advanced technology and scientific research.
Quantum dots structure
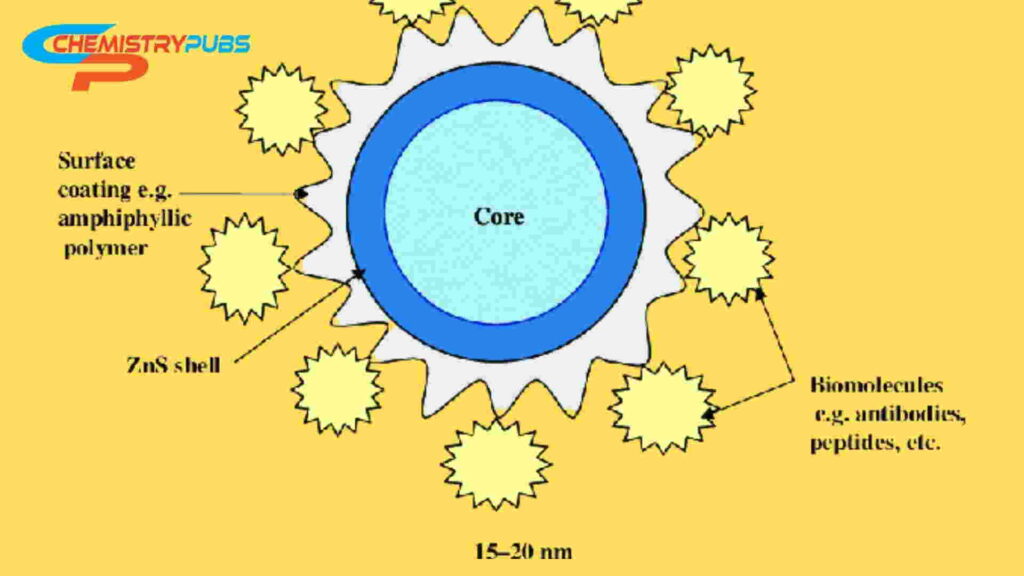
QDs structure consists of an inorganic core semiconductor material like CdTe or CdSe, and an inorganic shell of a different band gap such as ZnS. It is then overlapped by an aqueous organic coating to which biomolecules can be conjugated easily. It is very important to choose the shell and coating as the shell stabilizes the nanocrystal core. It also alters the photophysical properties at the time of coating. As a result, the achieving attractive properties make these crystals used in a wide range of applications like determining solubility in an aqueous media, providing reactive groups for binding to biological molecules, etc.

Quantum dots synthesis
QDS can be synthesized in two ways namely Top-Down and Bottom-Up approaches. In the top-down synthesis process, the bulk material must be thinned to make a quantum dot. In this process, electron beam lithography, reactive ion etching, focused beam lithography, and dip pen lithography techniques are used. There may be structural imperfections caused by patterning and contamination in QDs in this process.
In the bottom-up synthesis process relates to various chemical and physical methods to form QDs. The molecular beam epitaxial growth and colloidal synthesis methods are used in this process.
If we want to prepare CdSe QDs, then we must dissolve the 0.02 M Cd(Clo4)2.H20 salt into distilled water. Then the solution must be stirred and then thiol should be added as a stabilizer. Then NaOH solution should be added to control the pH level. The resultant solution is then deaerated by N2 bubbling for 30 minutes.
Solid Bulk Al2Te3 is then reacted with dilute sulphuric acid to generate H2Te gas. The produced gas with slowly regulated nitrogen is then introduced into the solution Cd-RSH precursor. Then the solution can be cooled down after exhausting the H2Te gas. Finally, we are able to get quantum dots after heating properly in the laboratory oven. The reactions for the synthesis of QDs are given below:
First Reaction
Cd2+ + H2Te —-à (HS-R) —à Cd-(SR)xTey+ 2H+
Second reaction
Cd2+ + NaHTe —à( HS-R) → Cd-(SR)xTe + H+ + Na+
Third Reaction
Cd-(SR)xTey –à(100˚C) CdTe Quantum Dots
Al2Te3 + H2SO4 –à Al2(SO4)3 + 3H2Te(gas)
If we want to synthesize Quantum dots by gas phase synthesis method, we will follow the following process:
This method is more economical than other methods. The QDs synthesis process is completed in plasma reactors, laser reactors, flame reactors, and inert gas reactors with the help of evaporating and condensing methods. The molecules can react easily in plasma reactors to form ionic forms resulting in nanoparticles/crystals/structures. Plasma reactor has a high cooling rate character. The same process can be achieved by using a laser instead of plasma. At that time, highly localized heating and cooling can be achieved easily.
This method uses gallium and Arsine (AsH3) as precursors. Ultrafine thin particles were created by using an aerosol generator. The selection of particles was completed easily by using a differential mobility analyzer. This tool works by following the principle of electrical mobility. As a result, smaller particle sizes can be achieved. Aerosol flow can be regulated before entering the reaction chamber to form perfect-size QDs nanocrystal diameter ranges from 1.5nm to 10 nm sizes.
QDs nanocrystals can be synthesized by applying the g LASER ablation technique. In this technique, highly energetic laser light is focused on metallic crystals or various crystals to create QD nanoparticles. One kind of nanosuspension is produced by ejected species. It may use Cu laser to obtain CdS/ ZnSe QDs by ablating CdS/ZnSe crystal in the open air under a thin layer of liquid above the surface of the suitable semiconductor.
The various liquid mediums namely DMSO, DETG, ethanol, isobutanol, and diethylene glycol can be used to obtain the QDs nanoparticles without the addition of any surface-active substances. An excellent suspension of CdS nanocrystals was then obtained in various liquid mediums. Then cooled and dried the synthesized QDs to get perfect nanocrystale.
Working Principle of Quantum Dots
There exist confined valence band holes and conduction band electrons in QDs. They have a distinct energy level that carries the electricity. The electrons in QDs have to occupy an energy level that ‘fits’ inside it. These emit photons at the time of excitation occurs. This phenomenon can be created by the QDs coming into contact with a light or electricity source. As a result, the longest wavelengths of light like red light are produced by the biggest QDs, and the shortest wavelengths of light like blue light are generated by the smallest QDs.
Properties of Quantum dots
Quantum dots contain artificial nanostructures that contain various attractive properties. The properties of QDs are determined by many factors like size, shape, composition, and structure.
Optical Properties of Quantum dots
QDs contain perfect optical properties. They possess intrinsic band gap. As a result, the electrons in QDs have the ability to transfer from the valence to the conducting band by absorbing incident light and producing a hole that can bind with electrons to each other to form an exciton. At that time, photons with longer wavelengths will be emitted when this exciton recombines. This phenomenon is generally called Fluorescence. The emission of wavelength from QDs completely depends on their size. The fluorescence phenomenon can be controlled easily by changing their size during the process of QD synthesis.
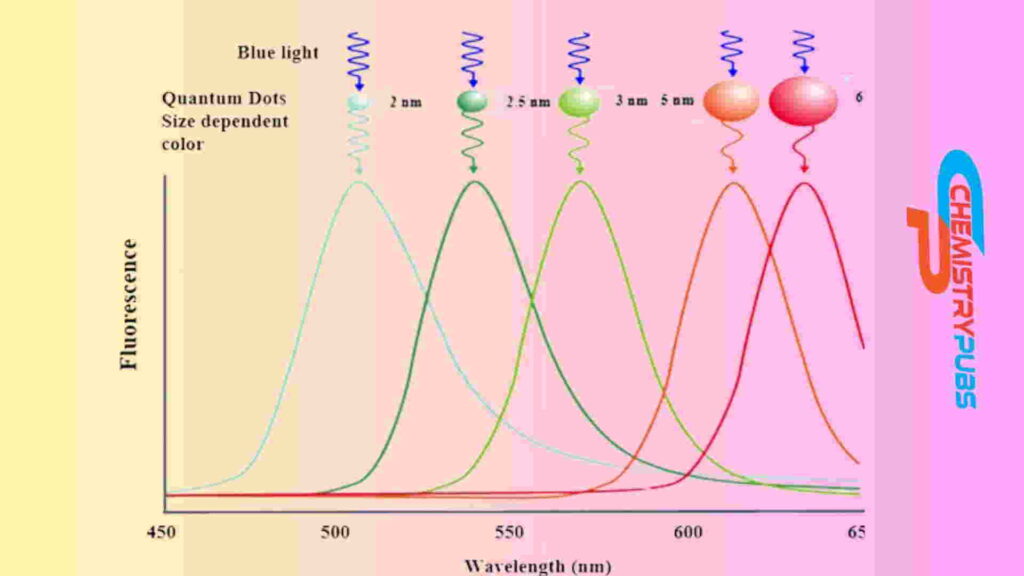
The emission of wavelengths phenomenon of quantum dots span is found from the UV to the infrared (IR).
QDs nanocrystal also contains high quantum yield, high photostability, and high molar extinction coefficient properties. The emissions from QDs nanocrystals are narrow and symmetrical at specific wavelengths.
Physical properties of Quantum dots
QDs nanocrystals contain a size not more than 10nm. They have tunable morphologies, excellent biocompatibility, high dispersibility, strong optical properties, and magnetism behavior. The size of QDs can be controlled which enables them to show strong and tunable fluorescent properties. Their attractive physical properties led to the discovery of QD materials such as CdS, PbS, silicon-based QDs, graphitic carbon nitride QDs, molybdenum disulfide QDs, and others.
Chemical properties of Quantum dots
The chemical properties of QDs relate to the chemical compositions of the core, shell, and ligands, which provide them with attractive properties to use in various fields. They show higher stability, less toxicity, good reactivity, and various thermodynamic and kinetic properties.
Types of Quantum dots
Quantum dots exist in various types based on their composition and structure. The types of quantum dots are discussed below:
Core-type Quantum dots
This type of QDs exists as single component materials with uniform internal compositions like cadmium, lead, or zinc. The photo- and electroluminescence properties of this type of QDs can be fine-tuned by simply changing the size.
Core-shell Quantum dots
The luminescent properties of these QDs arise from the recombination of electron-hole pairs through radiative pathways. The exciton decay of crystal can also occur through nonradiative methods for reducing the fluorescence quantum yield. It can form shells of another higher band gap semiconducting material around them to develop their efficiency and brightness of QDs. This type of QDs example is CdSe in the core and ZnS in the shell.
Alloyed quantum dots
This type of QDs contains compositions CdSxSe1-x/ZnS of 6nm diameter emits light of different wavelengths. It forms by alloying together two semiconductors with different band gap energies that exhibit interesting properties. This type of QD possesses novel and additional composition-tunable properties.
The Future of Quantum Dots
QDs contain near zero-dimensional and exhibit a sharper density. They have perfect optical and transport properties. As a result, they are used very much for potential uses in amplifiers, biological sensors, and diode lasers. They are very useful in cancer metastasis, embryogenesis, lymphocyte immunology, and stem cell therapeutics. Researchers also believe that QDs can be used as the inorganic fluorophore in intra-operative tumor detection when performed using fluorescence spectroscopy in the near future.
Applications of Quantum dots
Bioimaging
QDs have various applications in the field of biological analysis. The tiny particles of QDs allow them to go anywhere in the body. So they are perfect for biological applications such as medical imaging and biosensors. At present, they are used very much to know the intracellular processes, tumor targeting, in vivo observation of cell trafficking, diagnostics, and cellular imaging at high-resolution states.
QDs are considered superior to traditional organic dyes in many respects. The fluorescent probes made with QDs have to remain well-dispersed and stable in the aqueous medium with a wide range of pH and ionic strength measurement purposes during bioimaging applications.
Photovoltaic devices
QDs are used in various photovoltaic devices for having the tunable of the absorption spectrum properties and high extinction coefficient properties. It increases the efficiency of silicon photovoltaic cells and leads to reduced costs. They contain the largest bandgaps on top. It is possible to transmit the incoming photons until they reach a layer with a bandgap smaller than the photon energy.

Photodetectors
QDs can be used in integrated circuits. The QDs which are synthesized from solution-processed can be used for the integration of several substrates. The QDs which are synthesized from the colloidal method are used in machine vision, surveillance, spectroscopy, and industrial inspection.
Light-emitting devices
QDS are used in light-emitting diode (LED) making purposes. They contain unique optical properties. They have the properties of presenting visibly more accurate and outstanding colors.
Quantum computing
QDs are used to make powerful ‘supercomputers’ known as quantum computers. These computers are used quantum bits or ‘qubits’, which can exist in two states – both on and off simultaneously. These phenomena create the information processing speeds and memory capacity to both be greatly developed when compared to general computers.
Solar cell
QDs are used to make solar cells. They are used to replace bulky materials such as silicon, or copper indium gallium selenide. QDs contain bandgap which are essential for solar cells. QDs solar cell makes infrared energy (IR) as accessible as any other because most of the solar energy reaching the Earth is in the infrared region.
Biosensors
QDs are used to make various types of biosensors. Zinc sulfide-coated QDs are used for food toxin detection. There are many QDs-based biosensors used for monitoring pesticides. The phosphorus graphene QDs are used for the detection of NO2− in various samples.
Conclusions
QDs nanocrystals are very crucial elements nowadays. It contains outstanding properties for having a special size in nanometer ranges. It can be used in various applications in the fields of biological purposes, electronic purposes, chemical purposes, and so on. It helps to widen our knowledge in the fields of nanotechnology at present decades.
References
- https://www.nobelprize.org/uploads/2023/10/advanced-chemistryprize2023.pdf
- Mohamed, W. A., Abd El-Gawad, H., Mekkey, S., Galal, H., Handal, H., Mousa, H., & Labib, A. (2021). Quantum dots synthetization and future prospect applications. Nanotechnology Reviews, 10(1), 1926-1940.
- Cotta, M. A. (2020). Quantum dots and their applications: what lies ahead?. ACS applied nano materials, 3(6), 4920-4924.
- Joglekar, P. V., Mandalkar, D. J., Nikam, M. A., Pande, N. S., & Dubal, A. (2019). Review article on quantum dots: synthesis, properties and application. Int. J. Res. Advent Technol, 7(510.10), 32622.

