Cellulose applications are found in different types of industries like papers, fibers and textiles, cosmetics, and pharmaceutical industries. It is considered the most abundant biopolymer in the earth. Cellulose is the main structural component in herbal cells and tissues. There are many derivatives that can be synthesized from this organic compound like cellulose acetate, nitrocellulose, carboxymethyl cellulose, methylcellulose, hydroxypropyl cellulose, etc.
What is cellulose?
Cellulose is a very common complex carbohydrate that is constructed by oxygen, carbon, and hydrogen. This compound is chiral, tasteless, and has no odor. A French chemist Anselme Payen discovered this compound in 1838. Cellulose is completely biodegradable and less hygroscopic in nature. Cellulose applications cannot be described in a word. Cellulose has huge applications in different fields.
Cellulose definition
Cellulose is a natural bio-molecule consisting of β-d-1,4 glycosidic bonds. The molecular chain of this biopolymer is linear and makes it a good fiber-forming polymer. It is found in plant cell walls as well as in various other sources.
History of Cellulose
Cellulose biopolymer was first discovered by Anselme Payen in 1838. He also determined the chemical formula of cellulose molecules. Hyatt Manufacturing Company produced a thermoplastic material in 1870 namely celluloid. An important compound, namely rayon in the 1890s and cellophane in 1912 was produced from cellulose molecules. The chemical structure of this versatile biopolymer was discovered by Hermann Staudinger in 1920. Kobayashi and Shoda synthesized cellulose in 1992 without using any biological enzymes.
What are the sources of cellulose?
Cellulose is obtained from fruits, vegetables, agro wastes, agro residues, stalks, leaves, plant roots, etc.
Cellulose sources
The sources of cellulose are divided into two categories namely natural and synthetic. The natural cellulosic fibers come from mostly cotton, jute, flax, ramie, sisal, and hemp. Cotton and kapok sources contain 90% cellulose. Sisal, fique, agave fiber contain 33% cellulose. Flax, jute, kenaf, Hemp, ramie, and rattan fiber contain 33% cellulose. Fruit fibers contain 30-50% cellulose molecules. Rice, barley, wheat, straws, bamboo, and grass contain 40-50% cellulose molecules.
What is cellulose fiber?
Cellulose or cellulosic fiber is a type of fiber that is obtained from cellulose molecules. Cellulose fiber consists of a matrix of hemicelluloses impregnated with lignin molecules. There exist different types of cellulose fibers like flax fibers, cotton fibers, bast fibers, etc.
Cellulose fibers
They are regenerated from natural cellulose which comes from various sources. These sources are beech trees, bamboo fiber, etc. Papaya fiber, sugarcane bagasse, rice straw, wheat straw, etc. fiber are important cellulosic sources.
How cellulose is made?
This abundant organic compound is produced in plants through the process of photosynthesis. The molecules of carbon dioxide and water convert into glucose and oxygen after consuming energy from sunlight. It is the main structural component of plant cell walls. The molecules of glucose can be converted into cellulose by enzymes called cellulose synthases. The chains of cellulose molecules are connected together to form a good structure that gives plants their shape and strength.
Cellulose in plants
The cellulose molecules are found in the plant cell walls and various parts like fruits, leaves, and vegetables. This molecule is very much present in various fibrous materials like cotton. This molecule
Cellulose is found in the cell walls of plants and their organs such as fruits, leaves, and vegetables. Cellulose is naturally present in fibrous materials such as cotton. It is an important structural component of both lower and upper plant cell walls.
What is the structure of cellulose?
Cellulose is a common biopolymer that is abundant in nature. It consists of long β-glucose chains that are connected by 1,4 glycosidic bonds. We know that β-glucose is an isomer of α-glucose which has the ability to create 1,4 glycosidic bonds, so consecutive β-glucose can be rotated at 1800 to each other. There are many hydrogen bonds available in this molecule which have the ability to create between the long chains because of the inversion of β-glucose molecules.
Cellulose structure
Cellulose polymer is constructed by repeating anhydrous glucose units (AGU). These repeating units are attached by acetal functionalities containing repeating units of cellulose OH groups. The anhydrous glucose units have covalent bonds. The hydroxyl groups (OH) in cellulose molecules can be easily modified by interacting with functional groups after reacting with various chemicals. As a result, a wide range of cellulose derivatives can be derived like cellulose acetate, nitrocellulose, carboxymethyl cellulose (CMC), etc. Anhydrous glucose units are responsible for creating various reactivity and are influenced by the various steric effects of reagents. This biopolymer can be synthesized biologically by the connecting of glucose molecules with the loss of one H2O molecule after the formation of each bond.
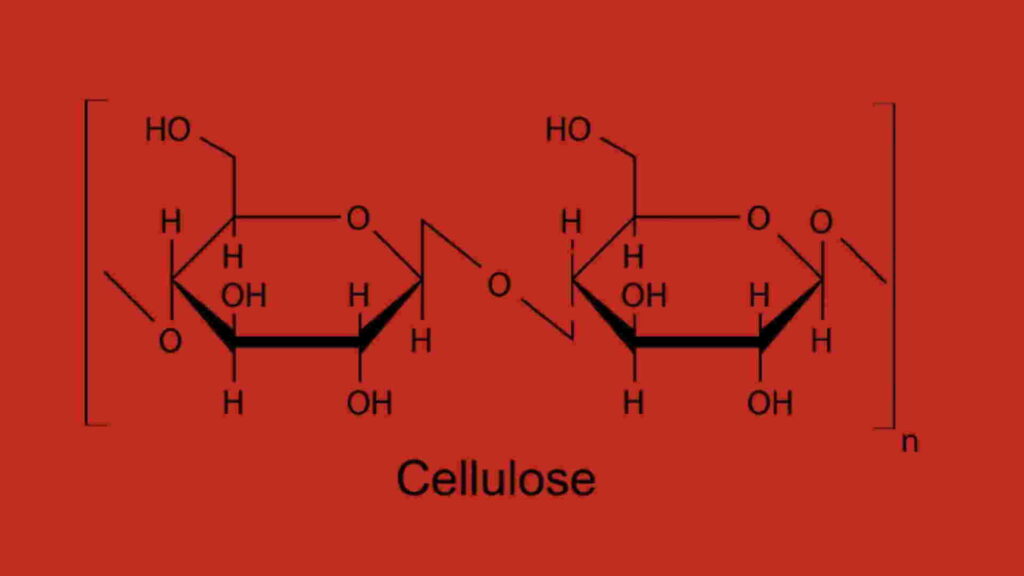
Cellulose structure formula
Cellulose is a crucial structural component of green plant cell walls. This compound can also be found in algae, acetobacter, and rhizobium, among other organisms. This biopolymer consists of a linear chain of several hundred to many thousands of β (1→4) linked D glucose units. This compound can be broken down into glucose after treatment with concentrated mineral acids like hydrochloric acid, nitric acid, sulphuric acid, etc. It is more crystalline than starch. It needs 320 degrees and a pressure of 25 megapascals to convert the crystalline state to amorphous state.
What are the functions of cellulose?
Cellulose is very important for plants to provide strength, cell division facilities, and definite structure.
This biopolymer is considered the structural protein in plants and algae. It helps to support plant cell walls. It acts as the reinforcing bars and the lignin acts like concrete.
Functions of Cellulose
Cellulose molecule is the main structural component of the plant cell walls.
Cellulose molecule has a higher tensile strength which enables it to be more stretched without breaking in plants.
Cellulose with lignin in plant cell walls creates a matrix that increases the strength of the cell walls in plants.
Cellulose helps plants to grow upright.
Cellulose is essential for the cell division of plants.
What are the properties of cellulose?
Cellulose molecules a nontoxic, biodegradable, biocompatible, and less hygroscopic in nature. Cellulose cannot dissolve into water molecules. Cellulose contains lower density but higher tensile strength. The cellulose molecule is white crystalline without any odor.
Cellulose properties
Cellulose is considered a renewable and biodegradable natural polymer. It has a lower density, high porosity, and a large specific surface area. The density of cellulose is 1.5 gm/cm3. The melting point of cellulose is 260-270oC. The molecular weight of cellulose is 162.1406 g/mol. It has no odor, no color, biocompatibility, negligible toxicity, and mild immune response.
Cellulose synthesis
At first, the natural cellulosic fibers are immersed in dirty water for bacterial degradation. Then some days later, the fibers are extracted from these sources. Then the extracted fibers are treated with detergent to remove dirt. Then it was treated with NaOH to separate the fibrous mass. Then the fibrous mass was treated with hydrogen peroxide (H2O2) to remove coloring components. Then it was treated with Sodium chlorite to remove the lignin. After that, it was treated with 0.2% sodium metabisulphate solution. Then, finally, it must be treated with 17.5% NaOH solution to remove the hemicelluloses. Then, the fiber should be washed properly and dried to get the pure cellulose.
Cellulose derivatives
There are various cellulose derivatives can be obtained by different chemical reactions with cellulose. The common cellulose derivatives are methylcellulose, cellulose acetate, nitrocellulose, hydroxypropyl cellulose, and carboxymethyl cellulose (CMC).
Cellulose ether derivatives
Cellulose ether derivatives contain higher molecular weight, special chemical structure and distribution of the substituent groups, degree of substitution, and molar substitution. These are obtained by replacing the hydrogen atoms of hydroxyl groups in the anhydroglucose units of cellulose with alkyl or substituted alkyl groups. These have good viscosity in solution, surface activity, thermoplastic film characteristics, and stability against biodegradation, and oxidation. The most obtained cellulose ether derivatives are methyl cellulose, hydroxyethyl cellulose, ethyl cellulose, hydroxypropyl cellulose, hydroxypropyl methylcellulose, carboxymethyl cellulose, and sodium carboxymethyl cellulose.
Cellulose ester derivatives
There are various cellulose esters obtained by treating the cellulose molecules with different chemicals. The most common cellulose ester derivatives are cellulose acetate, cellulose acetate phthalate, Cellulose acetate butyrate, Cellulose acetate trimelitate, hydroxypropyl methylcellulose phthalate, and so on. Cellulose ester derivatives have good film-forming characteristics which are widely used in the pharmaceutical field for controlling release preparations such as osmotic and enteric-coated drug delivery systems.
What are the properties of cellulose derivatives?
There are various attractive properties of cellulose derivatives. They show good physical aging, swelling behavior, sol-gel transition, or gel point. They also show attractive yield points, rigidity, and rupture strength.
What are the cellulose applications?
Cellulose is considered the most versatile and available natural polymer. It is widely used in different sectors like textile industries, biomedical applications, composite preparation, biofuel production, pharmaceutical industries, food industries, etc. for having attractive properties.
Cellulose derivatives
There are various cellulose derivatives namely cellulose acetate, methylcellulose, hydroxypropyl cellulose, nitrocellulose, and carboxymethyl cellulose (CMC). They are produced from cellulose after some chemical reactions with various chemicals.
What is cellulose acetate?
Cellulose acetate (CA) is a cellulosic derivative consisting of hydroxyl groups partially or completely acetylated. Cellulose acetate is insoluble in water, a nontoxic, nonirritant, and biodegradable material. Cellulose acetate has the property of heat resistance. CA is less hygroscopic in nature.
Cellulose Acetate
Cellulose acetate (CA) is one kind of ester that contains acetyl content ranging from 29.0% to 44.8%. They are divided into three types namely mono-, di-, and triacetate. Cellulose acetate is widely used for the production of semipermeable coating on tablets. It can be used to make osmotic pump-type tablets and microparticles for controlled release of drugs.
Synthesis of Cellulose acetate
This ester can be obtained by treating the cellulose molecule with acetic anhydride using a catalyst like sulfuric acid in a solvent. This compound is very safe for living organisms having high resistance to sunlight (in particular, ultraviolet rays).
Properties of Cellulose acetate
Cellulose acetate has a higher melting point and is carbonized at 220 to 300℃. CA shows lower electrical conductivity and large internal and external resistance. So, cellulose acetate can be used as an insulation material. Cellulose acetate has no odor and is less hygroscopic in nature. Cellulose acetate is not soluble in water. Cellulose acetate can be dissolved in an organic solvent like DMSO.
Applications of Cellulose acetate
Cellulose acetate can be used to make sunglass frames.
Cellulose acetate can be used to make filter media.
Cellulose acetate can be used to make cigarette filters.
Cellulose acetate can be used to make different types of textile fiber.
Cellulose acetate can be used to make a drug delivery system.
Cellulose acetate can be used to make composite membranes.
What is methylcellulose?
Methylcellulose is an important cellulose derivative that is hydrophilic in nature. Methylcellulose is used as a bulk-forming and stool-softening agent for the treatment of constipation. It can be used to treat the diseases of diarrhea associated with diverticulosis and irritable bowel syndrome. This derivative is essential to the proper maintenance of obesity and is administered topically to soften hard contact lenses. This derivative compound has a crucial role in acting as an emulsifier and stabilizer in various food industries.
Methylcellulose
Methylcellulose or MC is one kind of methyl ether of cellulose. This cellulosic derivative is produced by reacting methyl chloride and alkali cellulose molecules at a definite temperature. The MC molecule consists of 27.58%–31.5% of methoxy groups. MC compound is used very much in pharmaceutical industries to prepare oral solid pharmaceutical formulations as a binder and as a coating agent.
Properties of Methylcellulose
Methylcellulose has the properties of a binder with good plastic flow and wetting ability. It exhibits granular properties that compress easily and tablets with moderate hardness.
Methyl cellulose is soluble in water when heated up to 50°C. So, it is slightly soluble in water. The aqueous solution of methylcellulose shows thermal gelation properties at higher temperatures. It can be possible to make a hydroalcoholic solution of methylcellulose by dissolving it with various organic solvents such as ethanol and methanol with a small amount of water as a co-solvent.
What is carboxymethyl cellulose?
Carboxymethyl cellulose or CMC is a type of cellulose derivative that contains carboxymethyl groups in the glucopyranose monomers. It is considered a crucial cellulose ether derivative. It can be synthesized by treating the cellulose with alkylating reagents after activating the noncrystalline regions of cellulose molecules.
Carboxymethyl cellulose or CMC
Carboxymethyl cellulose or CMC consists of carboxymethyl groups. This compound can be synthesized after the reaction of a cellulosic molecule with chloroacetate in alkali to produce substitutions in the C2, C3, or C6 positions of glucose units.
Properties of Carboxymethyl cellulose (CMC)
Carboxymethyl cellulose or CMC exhibits water solubility.
Carboxymethyl cellulose or CMC is amenable to the hydrolytic activity of cellulases.
Carboxymethyl cellulose or CMC has flammable characteristics.
Carboxymethyl cellulose or CMC is a nontoxic molecule.
Carboxymethyl cellulose or CMC is a biodegradable, and renewable polymer.
Applications of Carboxymethyl cellulose (CMC)
Carboxymethyl cellulose or CMC is used to make hydrogel.
Carboxymethyl cellulose or CMC is used to make different types of films.
Carboxymethyl cellulose or CMC is used to make scaffold or nonwoven mats.
Carboxymethyl cellulose or CMC is used as a suitable filler in composites that enhances the mechanical properties.
Carboxymethyl cellulose or CMC is used in food packaging.
Carboxymethyl cellulose or CMC is used in several drug delivery and tissue engineering purposes in the field of medicine.
What is Nitrocellulose?
Nitrocellulose or cellulose nitrate is a common cellulose derivative. Nitrocellulose compound is highly flammable and is made up of nitric esters of cellulose. This derivative is used as modern gunpowder and in some lacquers and paints.
Nitrocellulose
Nitrocellulose or cellulose nitrate was first synthesized in 1845 by Schonbein. This biopolymer consisting of glucose subunits and hydroxyl groups is replaced by a nitro group. The strong hydrogen bonds are available in this derivative. This derivative can be obtained by treating the cellulose molecule with nitric acid in the presence of a sulfuric acid catalyst and water.
Nitrocellulose synthesis
A definite amount of cellulose is mixed with a mixture of concentrated nitric acid and sulfuric acid. Then 60oC heat is applied for 3 hours to replace the hydroxyl groups of cellulose into nitro groups. Finally, nitrate ester can be obtained which may refer to mono-nitrocellulose, di-nitrocellulose, tri nitrocellulose based on the reaction progress.
Nitrocellulose properties
Nitrocellulose has flammable properties. They do not aggregate by hydrogen bonding because they have fewer OH groups than the parent cellulose. Nitrocellulose is insoluble in water and does not dissolve in mineral acids. Nitrocelluse is less hygroscopic in nature.
Nitrocellulose Applications
Nitrocellulose is very much used to make particle-free filtrates in the reverse direction.
Nitrocellulose can be used in topical skin applications like liquid skin and the application of salicylic acid. These are active ingredients in Compound W wart remover.
Nitrocellulose is used to make jettison components of the rocket/space capsule and deploy recovery systems.
Nitrocellulose can be used as a finish on guitars and saxophones.
Nitrocellulose can be used as an aircraft dope. That was essential to paint onto fabric-covered aircraft to tighten and secure the content.
Nitrocellulose can be used to cover playing cards.
The lacquer from this compound can be used to make nail polish because it is cheap and dries easily.
The lacquer from the nitrocellulose compound can be used to coat aluminum or glass discs. It can used to make phonograph albums.
Nitrocellulose is used to make table tennis balls, guitar sticks, and certain photographic films.
Cellulose applications
There are numerous applications in cellulose. Cellulose applications can be found in food industries, pharmaceutical industries, textile industries, defense industries, etc. for having various versatile properties. The descriptions are given below:

Cellulose applications in the food industry
Cellulose biopolymer is used very much in food industries. It is an inexpensive source and can be used very much for the manufacture of foods. Cellulose and its derivatives are found in white bread with added fiber, vegetarian burgers, chicken nuggets, pre-shredded cheese, low-fat ice cream, and various food items.
Cellulose biopolymer is very much used in sauces for increasing its thickening and emulsifying actions. The thickening characteristics of this biopolymer allow for more air to be whipped into products such as ice creams. It has the ability to absorb moisture and coat ingredients in a fine powder. There are various anti-caking applications of this compound for having the characteristics of moisture-absorbing properties.
Cellulose applications in Medical
This important biopolymer is used to treat renal failure during the failure of the human kidney. Cellulose is widely used for wound dressing because it helps to moisten the environment around the wound, remove excess exudates, control definite temperature and pH, and control the pain from the wound. It is very much used in tissue engineering, controllable drug delivery systems, blood purification, etc.
Cellulose applications in textile industries
Cellulosic fibers are used to make various fabrics. Cellulose fibers have the properties of adsorbing moisture. It helps to remove the growth of bacteria with respect to the synthetic fabrics. Cellulosic fibers are used to make chemical filters and as fiber-reinforcement composites. Biocomposites and various polymeric composites can be processed by using the cellulose.
Cellulose applications in paper industries
Cellulose is used to make paper and paper-related products. At first, the chemical-treated pulp is produced from cellulosic fibers. Then it must used to make paper after some processing.
Cellulose applications in pharmaceutical industries
Microcrystalline cellulose (MCC) is widely used in the pharmaceutical industry due to having excellent properties. It can be used to make capsule coating and various types of drug delivery systems. Cellulose derivatives namely ethyl cellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, methylcellulose, carboxymethyl cellulose (CMC) or hydroxypropyl methylcellulose (HPMC), and anionic ether derivatives like sodium carboxymethyl cellulose (NaCMC) are widely used to make bioadhesives which are important to prepare buccal, ocular, vaginal, nasal and transdermal formulations.
What are the differences between cellulose and hemicelluloses?
There are various differences between cellulose and hemicelluloses which are discussed below:
Cellulose vs hemicelluloses
Differences between cellulose and hemicelluloses on the basis of definition
Cellulose definition: Cellulose is a biopolymer consisting of Anhydro glucose units (AGU). These units are connected by covalent bonds via bita 1,4-glycosidic linkages.
Hemicelluloses definition: Hemicelluloses is a kind of polysaccharide that consists of a number of heteropolymers like arabinoxylans which present along with cellulose in plant cell walls.
Differences between cellulose and hemicelluloses on the basis of structure
Cellulose: It is an un-branched biopolymeric molecule that contains 7,000–15,000 glucose molecules per polymer.
Hemicelluloses: It is a branched polymer that contains 500–3,000 glucose units.
Differences between cellulose and hemicelluloses on the basis of stability
Cellulose: It contains amorphous and crystalline regions. As a result, the strength of this biomolecule is higher than hemicelluloses.
Hemicelluloses: It contains amorphous regions. As a result, the strength of this biomolecule is lower than the cellulose molecule.
Differences between cellulose and hemicelluloses on the basis of subunits
Cellulose: It contains D-Pyran glucose units.
Hemicelluloses: It contains D-Xylose, mannose, L-arabinose, galactose, and glucuronic acid.
What are the differences between cellulose and lignin?
There are various differences between cellulose and lignin which are discussed below:
Cellulose vs lignin
Difference between cellulose and lignin on the basis of definition
Cellulose definition: Cellulose is an important polysaccharide consisting of hundreds to thousands of glucose units.
Lignin definition: Lignin is a complex cross-linked polymeric molecule that contains different amounts of three monolignols, namely coniferyl alcohol, p-coumarylalcohol, and sinapyl alcohol.
Difference between cellulose and lignin on the basis of water absorption properties
Cellulose: It is hydrophilic in nature.
Lignin: It is hydrophobic in nature.
Difference between cellulose and lignin on the basis of location
Cellulose: It is found in the primary cell wall of plants.
Lignin: It is found in the secondary cell wall of plants.
Difference between cellulose and lignin on the basis of structure
Cellulose: It contains a linear structure with linear β glucose chains.
Lignin: It has three-dimensional structural shapes.
Difference between cellulose and lignin on the basis of bonds
Cellulose: It contains hydrogen bonds or β 1-4 glycosidic.
Lignin: It contains ester bonds or ether bonds.
What are the differences between cellulose and starch?
There exist various differences between cellulose and starch which are discussed below:
Cellulose vs starch
Difference between cellulose and starch on the basis of definition
Cellulose definition: It is a polysaccharide composed of β glucose subunits linked together by glycosidic linkages.
Starch definition: It is a carbohydrate consisting of glucose subunits joined together by glycosidic linkages containing two components namely amylase, and amylopectin.
Difference between cellulose and starch on the basis of glucose range
Cellulose: It contains 100-10,000 glucose subunits.
Starch: It contains 200-1000 glucose molecules.
Difference between cellulose and starch on the basis of function
Cellulose function: It provides the structural support and strength to plants.
Starch function: It is considered the main carbohydrate storage in plants.
Difference between cellulose and starch on the basis of linkage
Cellulose linkage: It contains beta linkage.
Starch linkage: It contains alpha linkage.
Difference between cellulose and starch on the basis of chain type
Cellulose chain type: It contains a straight-chain polymer.
Lignin chain type: It contains a long and branched chain.
Why can’t humans digest cellulose while ruminants can?
Cellulose cannot be digested by humans because of the absence of appropriate enzymes (cellulase) to break down this polymeric molecule. The human guts do not have the bacteria that extract cellulase enzyme to digest the cellulosic fiber. So, humans cannot digest cellulose.
Animals can digest cellulose because of the presence of bacteria in their guts. These bacteria extract cellulase enzymes to digest the cellulosic fiber.

