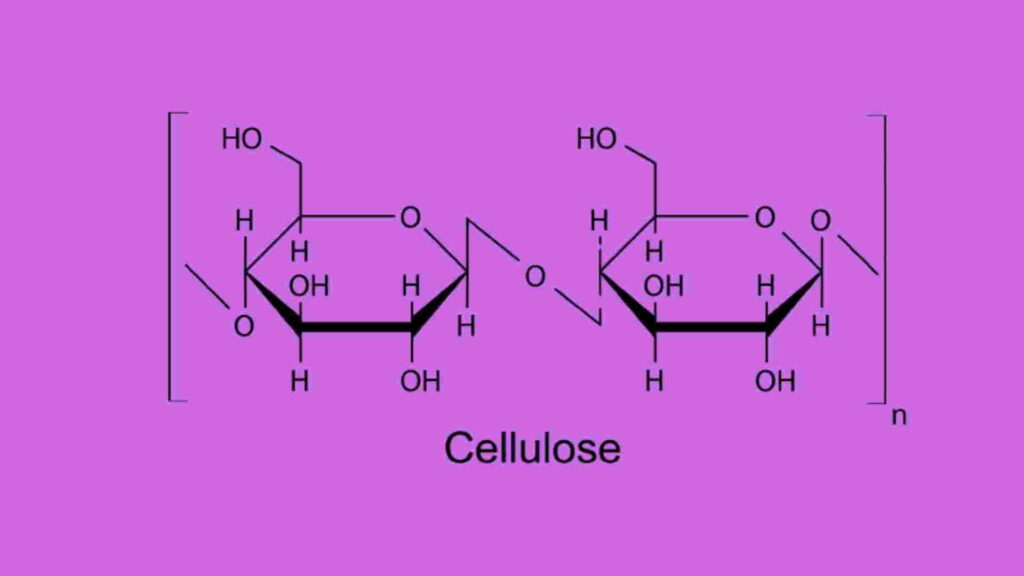
Cellulose structure is concerned with the connection of a linear chain of glucose molecules linked together through an oxygen atom covalently bonded. This bond has a good linkage to C1 of a glucose molecule and C4 of the adjacent molecule.
Cellulose structure has a C4-OH group at the non-reducing end and a C1-OH group at the reducing end with an aldehyde structure. There are extra carbonyl and carboxy groups in technical celluloses. This molecular structure of cellulose is responsible for showing various attractive properties like chirality, hydrophilicity, degradability, and chemical variability for having the donor group—OH.
Cellulose structure mainly focuses on the β(1→4)-glycosidic bonds between D-glucose units. The connection between the glucose monomers makes it a straight-chain polymer where the hydroxyl groups on the glucose monomers form hydrogen bonds with oxygen atoms and hold the chain in place to make high tensile strength to the fibers.
Cellulose structure is responsible for creating outstanding properties like hydrophilic, insoluble in water, and biodegradability in nature. It has a 467 degrees Celsius melting point and can be degraded into glucose by acid treatment at high temperatures.
What is cellulose?
Cellulose is considered the most plentiful polysaccharide on our planet which has a chemical formula [(C6H10O5)n]. It consists of glucose units that are connected by glycosidic linkage. Cellulose bio-molecule is generally synthesized from different plants and various agricultural residues.
Cellulose definition
Cellulose is a common but complex polysaccharide. Cellulose is considered the main component of the plant cell wall and is found mostly in plants mainly in the leaves and stalks. Cellulose structure has a linear chain consisting of d-glucose subunits linked by β (1–4)-glycosidic bonds. Cellulose is present in plant cell walls with hemicelluloses lignin and a small amount of extractives. Cotton fiber and wood from forests are the main sources of this bio-molecule for industrial applications.
Cellulose is used very much in textile industries, pharmaceutical industries, biomedical fields, food industries, and paper industries for having versatile properties. It is possible to make various derivatives from this biomolecule after some chemical modifications. The common cellulosic derivatives are cellulose acetate, nitrocellulose, methylcellulose (MC), hydroxyethyl cellulose (HEC), carboxymethyl cellulose (CMC), hydroxypropyl methylcellulose (HPMC), hydroxypropyl cellulose (HFC), ethyl hydroxyethyl cellulose (EHEC), etc.
What are the examples of cellulose?
Cellulose is obtained from wood, cotton, wool, stalk, fruit fibers, stalk fibers, leaf fibers, and bast fibers after some chemical modifications.
What are the sources of cellulose?
Cellulose bio-molecules can be synthesized from seed fibers, fruit fibers, stalk fibers, leaf fibers, and bast fibers from natural plants.
Cellulose sources
Cellulose bio-molecules can be synthesized from cotton and kapok fiber that contain 90% cellulose.
Cellulose bio-molecules can be synthesized from leaf fibers like fique, sisal, and agave that contain 33% cellulose.
Cellulose bio-molecules can be synthesized from bast fibers like kenaf, Hemp, ramie flax, jute, rattan, and vine fiber that contain 30% cellulose.
Cellulose bio-molecules can be synthesized from fruit fibers like coir fibers that contain 30-50% cellulose.
Cellulose bio-molecule can be synthesized from stalk fibers like barley, wheat straws, bamboo, Rice, grass, Tree wood fibers, rice, barley, wheat straws, and bamboo, grass, and tree wood that contain 40-50% cellulose.
How much cellulose is produced per year?
Cellulose is considered the most available biomass material on Earth which produces 1.5 x 1012 tons per year worldwide. It requires 0.5 billion tons for the production of paper and 1.5 billion tons of cellulose is used by humans and animals as food materials.
What is cellulose used for?
Cellulose molecule is used to prepare various cellulosic derivatives like cellulose acetate, nitrocellulose, carboxymethyl cellulose, ethyl cellulose, amino cellulose, etc. Cellulose bio-molecule is widely used to make paper and paper products. It is used in pharmaceutical industries to make various drugs. Cellulose molecules can be used as an important supplement in the diet.
What is cellulose structure?
Cellulose structure consists of linear chains of glucose units which are connected by β-1,4-glycosidic bonds. These chains are attached via hydrogen bonds and van der Waals forces to make a long thread-like crystalline structure. These are called cellulose microfibrils.
Cellulose structure
Cellulose structure contains linearly linked anhydrous glucose monomers through glycosidic linkage. There exist three hydroxyl groups in each repeating unit of glucose monomer. These hydroxyl groups contain hydrogen bonds which are the main factors to express the outstanding physical properties of cellulose molecules. The interchain of this bonding between hydroxyl groups and oxygens of the adjoining ring molecules stabilizes the total linkage. So, a linear configuration of cellulose molecules can be achieved.
The intermolecular hydrogen bonds and van der Waal forces between hydroxyl groups and oxygens of adjacent molecules in cellulose structure are responsible for making the proper cellulose chain fibrils. As a result, a highly ordered crystalline cellulose structure and regions among this bio-molecule can be achieved.
Cellulose bonds
Cellulose structure contains d-glucopyranose units linked together by β-1,4-glycosidic bonds. It contains higher molecular weight with a DP (degree of polymerization) sometimes exceeding 10,000.
Cellulose structure linkage
Cellulose structure consists of a beta-acetal linkage among the glucose chains. The monomer of glucose is beta-D-glucose, and all the beta-acetal links connect the number 1 carbon one glucose to the number 4 carbon of the next glucose molecule.
Cellulose glycosidic linkage
The glucose monomers in cellulose structure are linked together in unbranched chains by β-1,4 glycosidic linkages. These glucose monomers are flipped relative to the next one forming in a linear and fibrous structural shape.
Cellulose structure diagram
Cellulose IUPAC name
The cellulose IUPAC name is given below:
(6S)-2-(hydroxymethyl)-6-[(3S)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxane-3,4,5-triol.
Cellulose Molecular Weight
Cellulose molecular weight is given below:
342.30 g/mol
Cellulose Chemical Formula
The cellulose chemical formula is given below:
(C6H10O5)n
Cellulose density
The cellulose density is given below:
Density= 1.450 g/cm3
Cellulose Boiling Point
Cellulose Boiling Point is given below:
Boiling Point=527.1ºC at 760 mmHg
Cellulose Flash Point
The cellulose flash point is given below:
Flash Point= 286.7ºC
Cellulose molecular weight
Cellulose molecular weight is given below:
Molecular Weight/ Molar Mass= 162.1406 g/mol
Cellulose melting point
The cellulose melting point is given below:
How cellulose is synthesized?
Cellulose molecules can be synthesized by retting the source first. Then the extracted cellulose fibers are treated with some chemicals to get pure cellulose. These processes are bleaching and alkali treatments.
Cellulose synthesis
Cellulose can be synthesized easily from cotton, flax, hemp, jute, kenaf, sisal, and bamboo. Nowadays, researchers are trying to synthesize this biopolymer from different agricultural wastes like sugarcane bagasse, vegetable stems, rice husks, rice straws, wheat straws, etc.
The cellulose synthesis process is done by using steam explosion and xylanase pretreatment and bleaching process. At first, the various parts of the plants are kept in dirty water for about 2 weeks. After retting, the fibers can be extracted mechanically or by hand. Then the extracted fibers are washed with detergent to remove dirt. Then the fibers are treated with hydrogen peroxide (H2O2) to remove the coloring materials.
Then the bleached fibers are treated with 0.7% sodium chlorite (NaClO2) solution adjusted to a pH of 4 by the addition of weak acetic acid at 70°C for 1 hour. These chemicals help to remove the coloring materials from the fiber. Then the fiber must be washed again and again with distilled water until the pH of the water is neutral and dried at 55°C for 24 hours. Then the fibrous masses are treated with 17.5% NaOH after treatment with 0.2% sodium metabisulfite solution. Finally, we get the pure cellulose.
Cellulose Biology
Cellulose Biology focuses on the cellulose structure which consists of linearly linked glucose monomers by β-1,4 glycosidic linkages. The main sources of cellulose are cotton, flax, hemp, jute, kenaf, sisal and bamboo, sugarcane bagasse, vegetable stem, rice husk, rice straw, wheat straw, etc. Cellulose can be synthesized after retting, alkali treatment, and bleaching process. Cellulose has higher strength, non-toxicity, and biodegradable properties. Cellulose molecule is formed by the biosynthesis process.
What are the applications of cellulose?
Cellulose has a wide range of applications. The natural fibers from cellulose are used very much in textile industries. There exist various plant fibers like sisal, jute, and hemp in the market, and cellophane, rayon, and other “regenerated cellulose fibers” have a small portion in the market. This important compound is used to make paperboard, paper, and card stock. There are various electrical insulating materials in cables, transformers, and other electrical equipment are constructed by this compound partially.

Cellulose applications
This compound creates vast applications in the fields of the pharmaceutical industry, food industry, nanotechnology, cosmetics, and drug-delivery systems in treating cancer and other diseases. Microcrystalline cellulose (MCC) can be used in the food, cosmetics, pharma industry, etc. for having its binding and tableting properties, characterized by its plasticity and cohesiveness when wet.
Cellulose uses in the food industry
Cellulose is widely used in food industries for having excellent properties like high surface area, high water-holding capability, rheological properties, and biocompatibility. It contains outstanding stabilizing, bulking, fluid, properties, and higher water-holding capability. So, cellulose molecule is demonstrated as a promising low-calorie bulking ingredient to enhance the functional foods of different forms.
Cellulose uses in the pharmaceutical industry
Cellulose is used in pharmaceutical industries for having versatile properties. Cellulose is used very much in the pharmaceutical fields to prepare various oral solid dosage forms known as capsules and as a bulking agent to develop the mass in formulations containing small amounts of active ingredients. This biomolecule is used to make powder dosage forms. It acts as a suspending agent for aqueous peroral delivery and an adsorbent. It is used very much in pharmaceutical fields to make thickening agents for topic preparations. The microcrystalline cellulose is very much used to make various oral suspensions.
Cellulose uses in textile industries
Cellulose application in textile industries can never be described in a word. It is used to make various types of yarns and fabrics. It is also used to coat various fibers, yarns, and fabrics. As a result, it can be obtained the proper thickness and bursting strength of the fibers, yarns, and fabrics and decrease the penetration of water through the composite fabrics and the water vapor permeability. The hydrophobicity and flexibility of this bio-molecule make it more outstanding to use it in different textile sectors.
What are the functions of cellulose?
Cellulose molecules are essential to plant growth. It provides essential strength and helps to upright growth. It plays a crucial role in plant cell division.
Functions of cellulose
Cellulose increases the flexibility of plants.
Cellulose provides accurate strength to plants.
Cellulose provides the necessary mechanical properties to plants.
Cellulose provides excellent structural support to plants.
Cellulose provides the proper rigidity to the cell walls.
Cellulose maintains plant shape and withstands mechanical stress.
Cellulose maintains the issue of various resistances to environmental factors such as wind and gravity.
Cellulose molecule has the greatest contribution to the development of the plant.
Cellulose bio-molecules act as a barrier against pathogens and pests.
Cellulose bio-molecules have defense characteristics against potential threats and help plants to resist infections.
What are the cellulose derivates?
Cellulose derivatives are produced from cellulose molecules after some chemical modifications. The most common cellulose derivatives are cellulose acetate, nitrocellulose, carboxymethyl cellulose, ethyl cellulose, amino cellulose, cellulose acetate butyrate, cellulose acetate triplicate, hydroxupropylmethyl cellulose phthalate, etc.
Cellulose derivatives
Cellulose derivatives comprise an important field in making various types of materials. They are synthesized from the cellulose bio-molecule by applying some chemical process. These derivatives are divided into two categories namely cellulose ether derivatives and cellulose ester derivatives.
Cellulose ether derivatives
The most common cellulose ether derivatives are ethyl cellulose, hydroxypropyl cellulose, hydroxyethyl cellulose, hydroxypropylmethyl cellulose, methylcellulose, etc. They have higher molecular weight and the proper distribution of the substituent groups. Cellulose ether derivatives contain higher viscosity in solution, excellent surface activity, thermoplastic film characteristics, and stability against biodegradation, and oxidation.
Cellulose ester derivatives
Cellulose ester derivatives are cellulose acetate, cellulose acetate butyrate, cellulose acetate phthalate, cellulose acetate trimelitate, and hydroxupropylmethyl cellulose phthalate. These derivatives are widely used to make various composite materials for have excellent film-forming characteristics.
What are the properties of cellulose derivatives?
Cellulose derivatives have attractive properties like good swelling behavior, sol-gel transition or gel point, rigidity, physical aging, and rupture strength.
What is cellulose acetate?
Cellulose acetate is a common ester derivative of cellulose that consists of hydroxyl groups partially or completely acetylated. Cellulose acetate molecule is regarded as a nontoxic, nonirritant, and biodegradable material.
Cellulose acetate
Cellulose acetate molecule can be synthesized by following the esterification or trans-esterification reaction process. The DS value of this derivative ranges from 29.0% to 44.8% and can exist as mono-, di-, and triacetate. Cellulose acetate is very much used to make cellulose acetate fibers, filter media, cigarette filters, osmotic pump-type tablets, sunglass frames, and microparticles for drug delivery systems.
Cellulose acetate applications
Cellulose acetate is used to make filter media, sunglass frames, synthetic fiber, cigarette filters, toys, playing cards, etc.
What is methylcellulose?
Methylcellulose is a very important cellulose derivative containing an alkyl group which is hydrophilic in nature. Methylcellulose can be synthesized after a proper reaction between methyl chloride and alkali cellulose molecule at a definite temperature.
Methylcellulose
Methylcellulose or Methylcellulose (MC) is the methyl ether of cellulose derivative. This molecule is synthesized by reacting methyl chloride and alkali cellulose. Methylcellulose or Methylcellulose (MC) contains 27 %–31.% of methoxy groups.
Methylcellulose applications
Methylcellulose or Methylcellulose (MC) is used mainly in pharmaceutical industries to prepare oral solid pharmaceutical formulations as a binder and as a good coating agent material for having good plastic flow and wetting ability.
What is carboxymethyl cellulose?
Carboxymethyl cellulose (CMC) contains carboxymethyl groups in the glucopyranose monomers. It is synthesized after proper reactions of cellulose molecules with alkylating reagents after activating the noncrystalline regions of cellulose molecules.
Carboxymethyl cellulose properties
Carboxymethyl cellulose (CMC) is a nontoxic molecule.
Carboxymethyl cellulose (CMC) is biodegradable.
Carboxymethyl cellulose (CMC) is a renewable polymer.
Carboxymethyl cellulose (CMC) is a flammable molecule.
Carboxymethyl cellulose applications
Carboxymethyl cellulose or CMC is used as a suitable filler in composites.
Carboxymethyl cellulose or CMC is used to make films.
Carboxymethyl cellulose or CMC is used to prepare hydrogel and scaffold.
Carboxymethyl cellulose or CMC is used to make food packaging.
What is Nitrocellulose?
Nitrocellulose or cellulose nitrate is an important cellulose derivative which can be produced Nitrocellulose or cellulose nitrate. This molecule was first synthesized in 1845 by Schonbein.
Nitrocellulose properties
Nitrocellulose is biodegradable and renewable. Nitrocellulose is a highly flammable and toxic material.
Nitrocellulose applications
Nitrocellulose is used to make gunpowder, filter media, table tennis balls, guitar sticks, certain photographic films, and in some lacquers and paints.
What are the differences between cellulose and hemicelluloses?
The main differences between cellulose and hemicellulose molecules are written below:
Difference between cellulose and hemicelluloses on the basis of definition
Cellulose definition: Cellulose is a linear-shaped biomolecule consisting of anhydrous glucose units joined by covalent bonds via bita 1,4-glycosidic linkages.
Hemicelluloses definition: Hemicellulose contains a number of heteropolymers like arabinoxylans and the presence of cellulose molecules.
Difference between cellulose and hemicelluloses on the basis of cellulose structure and Hemicellulose structure
Cellulose structure: Cellulose structure consists of 7,000–15,000 glucose monomers per polymer connected by glycosidic linkage.
Hemicellulose structure: Hemicellulose structure consists of 500–3,000 glucose units.
Difference between cellulose and hemicelluloses on the basis of stability
Cellulose stability: Cellulose biomolecule has more stability than Hemicellulose for having amorphous and crystalline regions.
Hemicellulose stability: Hemicellulose biomolecule shows lower stability than cellulose biomolecule for the absence of crystalline regions.
Difference between cellulose and hemicelluloses on the basis of monomer
Cellulose monomer: Cellulose biomolecule consists of D-Pyran glucose monomers.
Hemicellulose monomer: Hemicellulose monomers are D-Xylose, mannose, L-arabinose, galactose, and glucuronic acid.
What are the differences between cellulose and lignin?
The main differences between cellulose and lignin molecules are written below:
Difference between cellulose and lignin on the basis of definition
Cellulose definition: Cellulose has hundreds to thousands of glucose subunits connected by covalently glycosidic linkages.
Lignin definition: Lignin is an important cross-linked bio-polymeric molecule having three monolignols namely coniferyl alcohol, p-coumarylalcohol, and sinapyl alcohol.
Difference between cellulose and lignin on the basis of water absorption properties
Cellulose absorption property: Cellulose absorption property is higher.
Lignin absorption property: Lignin has no absorption property because it has a hydrophobic character.
Difference between cellulose and lignin on the basis of location
Cellulose location: It is found in the primary cell wall of plants.
Lignin location: It is found in the secondary cell wall of plants.
Difference between cellulose and lignin on the basis of structure
Cellulose structure: It contains a linear shape structure having β glucose subunits connected by glycosidic linkage.
Ligninb structure: It has three-dimensional structures for having coniferyl alcohol, p-coumarylalcohol, and sinapyl alcohol.
Difference between cellulose and lignin on the basis of bonds
Cellulose bonds: Cellulose contains hydrogen bonds or β 1-4 glycosidic bonds.
Lignin bonds: Lignin contains ester bonds or ether bonds.
What are the differences between cellulose and starch?
The main differences between cellulose and starch molecule are written below:
Difference between cellulose and starch on the basis of definition
Cellulose definition: It is a common straight-chain polysaccharide composed of β glucose monomers linked together by glycosidic linkages.
Starch definition: It is a common carbohydrate consisting of amylase, and amylopectin joined together by glycosidic linkages
Difference between cellulose and starch on the basis of glucose range
Cellulose monomer: It consists of 100-10,000 glucose subunits.
Starch monomer: It consists of 200-1000 glucose molecules.
Difference between cellulose and starch on the basis of function
Cellulose function: It provides the structural shape and rigidity to plants.
Starch function: It is considered the main carbohydrate storage in plants.
Difference between cellulose and starch on the basis of linkage
Cellulose linkage: It consists of a beta linkage.
Starch linkage: It consists of alpha linkage.
Difference between cellulose and starch on the basis of chain type
Cellulose chain type: This biomolecule is a straight-chain polymer.
Lignin chain type: This biomolecule is a long and branched chain.
Why can’t humans digest cellulose while ruminants can?
Humans are unable to digest cellulose molecules because of the absence of enzymes (cellulase) to break down this polymeric molecule. On the other hand, ruminants can digest the cellulose molecule because they have cellulase enzymes to break down this polymeric molecule.
What are the properties of cellulose?
Cellulose is considered a non-toxic, bio-degradable polymer. It contains high tensile and compressive strength. Cellulose cannot dissolve in most of the organic solvents. Cellulose has no odor and is completely biodegradable. It contains chiral carbon. It needs 320 degrees and a pressure of 25 megapascals to convert crystalline to an amorphous transition state. This compound can be broken down into glucose at higher temperatures after treating with concentrated mineral acids.

Cellulose Properties
The properties of cellulose depend on the isolation process. The properties of this compound largely depend on the number of inter- and intra-molecular hydrogen bonds. The properties like chain length distribution, crystallinity, chain lengths, and the distribution of functional groups within the repeating units and along the polymer chains depend on the cellulose structure. Cellulose structure contains both crystalline and amorphous regions.
The FTIR band spectra at around 1430 cm-1 are associated with the amount of crystalline cellulose structure. The FTIR band spectra at around 898 cm-1 are associated with the amount of amorphous region in cellulose molecules. Cellulose properties are associated with the thermal stability issue which can be measured by a thermogravimetric analysis (TGA) machine.
The cellulose molecule is very stable up to 250oC. The properties of cellulose include having the values of good crystallinity index which is measured by X-ray diffraction method (XRD). The degradation of cellulose molecules is controlled by a phase boundary-controlled reaction
The molecular formula of cellulose is (C6H10O5)n
The molecular weight of cellulose is 162.1406 g/mol.
The density of cellulose is 1.5 g/cm3
The melting point of cellulose is 260-270oC.
The appearance of cellulose is a white powder.
What is Microcrystalline Cellulose (MCC)?
Microcrystalline cellulose or MCC is a crystalline cellulosic polysaccharide having β-1,4-glucosidic bonds synthesized from the α-cellulose molecule. Microcrystalline cellulose or MCC is obtained from fibrous plant material after proper acid hydrolysis of cellulose using 2M hydrochloric acid at 105 °C for 15-20 minutes.
Microcrystalline Cellulose (MCC)
Microcrystalline cellulose or MCC is one type of partially depolymerized cellulosic molecule. MCC can be synthesized by a reactive extrusion process, mechanical grinding process, ultrasonication process, enzyme-mediated process, steam explosion process, and acid hydrolysis process.
Microcrystalline Cellulose (MCC) properties
Microcrystalline cellulose or MCC is a non-toxic, less hygroscopic, crystalline, biocompatible, and biodegradable material. The size of MCC is lower than 5 μm must not be more than 10% and the DP is typically less than 400. The properties of Microcrystalline cellulose can be justified by the differential scanning calorimetry (DSC), Thermogravimetric analysis technique (TGA), and differential thermal analysis (DTA) techniques.
Microcrystalline Cellulose (MCC) applications
Microcrystalline Cellulose or MCC is used to make solid dose forms. MCC is widely used in food industries as an anti-caking agent, stabilizer, texture modifier, or suspending agent. MCC is also used in textile industries to make high-quality fabric and yarn. MCC is getting popular for making various bio-composites.
What is nanocellulose?
Nanocellulose is an advanced-character cellulose form that has a dimension in the nanoscale. It has excellent properties if we compare it to cellulose and its microfibers. Nanocellulose has a higher surface area, good tensile strength, flexibility, higher surface area, and abundant availability.
Nannocellulose
Nanocellulose or nano-structured cellulose is synthesized via acid hydrolysis of pure cellulose mostly using inorganic acids like H2SO4, HCl, etc. It can also be prepared by mechanical disintegration process in a high-pressure homogenizer an ultra-fine grinder, or a microfluidizer using high shear forces.
Nanocellulose preparation
Nanocellulose can be synthesized by the mineral acid hydrolysis with pure cellulose. The hydrogen ions from mineral acids come into the amorphous regions in the cellulose structure to break the 1,4-β-glycoside bonds. So, amorphous regions in cellulose structure are hydrolyzed easily after acid hydrolysis. Finally, the crystalline region in cellulose structure does not change and contains the ability to avert the permeation of the acid molecules.
There are various mineral acids used to synthesize the Nanocellulose. These mineral acids are sulfuric acid, hydrochloric acid, phosphoric acid, and hydrobromic acid.
It should be used 58% concentrated sulphuric acid or 2.5 Molar concentrated HBr acid or the mixture of sulfuric acid (98%, w/w), hydrochloride (37%, w/w), and H2O at a ratio of 3: 1: 6 (v/v) can be used at 60°C for 4 hours to get nanocellulose.
What are the properties of Nanocellulose?
Nanocellulose is considered a nontoxic and biodegradable polymeric molecule. It has a lower density but contains good electric conduction properties. It shows good biocompatibility and higher mechanical strength. Nanocellulsoe has good crystalline properties.
What are the applications of Nanocellulose?
Nanocellulose applications can never be described in a word. It is widely used in biomedical sectors, electronic sectors, food industries, textile sectors, composite preparations, etc. for having attractive properties.
Nanocellulose applications
Nanocellulose is considered the most interesting material for reinforcing plastic production. Nanocellulose is used to increase the various mechanical properties of thermosetting resins, starch-based matrixes, rubber latex, and soy protein. It can be reinforced with polyvinyl alcohol with boric acid to get fire retardancy properties.
Nanocellulose in the food industry
Nanocellulose can be used in food industries to produce food packaging material. Nanocellulose has excellent fiber-to-fiber bond strength. So, it can be used as a barrier in grease-proof type papers.
Nanocellulose in the pharmaceutical industry
Nanocellulose is used in pharmaceutical industries to make various antimicrobial films, tissues, and water-absorbent pads. Nanocellulose can be used to make solid dosages and used for drug delivery systems.
Nanocellulose in the textile industry
Nanocellulose is used in textile industries to make different types of fiber, yarn, and fabric. These products contain special strength and lower density.
Nanocellulose in the electronic industry
Nanocellulose is used in the electronic industry for having good conduction properties. It is used to make special type quantum dots which are very attractive terms among researchers nowadays.
Important Questions
What is cellulose made of?
Cellulose is an important biomolecule containing oxygen, carbon, and hydrogen atoms. Cellulose structure consists of the glucose subunits that are linearly linked by glycosidic linkage. The cellulose chemical formula is [(C6H10O5)n].
What is cellulose nanocrystal (CNC)?
Cellulose nanocrystal or CNC is a crystalline form of cellulose chain. The length and diameter of cellulose nanocrystal (CNC) compound is 200–600 nm and 3–40 nm respectively.
What is Cellulose nanofibril (CNF)?
Cellulose nanofibril or CNF is a crystalline cellulose that contains a crystallinity is about 90%. The diameter of Cellulose nanofibril or CNF ranges from 5 to 50 nm and the length is a few micrometers.
What is bacterial cellulose (BC)?
Bacterial cellulose or BC is microbial-type cellulose that is synthesized from various bacteria like Acetobacterxylinum bacteria.
What is lignin?
Lignin is one type of complex biopolymeric molecule having a group of aromatic alcohols namely p-coumarylalcohol, coniferyl alcohol, and sinapyl alcohol. The monolignols in the lignin molecule connect through several linkages in a three-dimensional network.
What is Hemicellulose?
Hemicellulose is a complex heteropolymer. It consists of various types of sugar molecules, including xylose, arabinose, mannose, galactose, and glucuronic acid. Hemicellulose has 500 to 3,000 sugar units which are connected with cellulose and pectin to form a cross-linked fibers network.
What is cellulose fiber?
Cellulose fibers are common fibers mostly derived from the bark, wood, or leaves of plants. Cellulose fiber consists of linear glucose subunits with the hemicellulose and lignin molecule. Cellulose fibers are used in textile industries, paper industries, and pharmaceutical industries, food industries for having excellent properties like higher strength, nontoxicity, biodegradability, and biocompatibility.
Cellulose fibers examples
Cellulose fibers are extracted from various natural sources like wood, grass, hemp, ramie, straw, flax, bamboo, or cotton. They are collected from various agro wastes like rice straw, wheat straw, sugarcane bagasse, rice husk, etc.
Cellulose fiber uses
Cellulose fibers are used in textile industries to make good quality fabric and yarn. Cellulose fibers are used in the food industry to make various food thickeners, emulsifiers, and stabilizers. Cellulose fibers are used in the paper industry to make good-quality paper products. Cellulose fibers are used in pharmaceutical industries to make solid dosages and drug delivery systems. Cellulose fibers are used in biomedical sectors to make various materials like wound dressing.
Is cotton a cellulose fiber?
Cotton fibers are considered the most pure form of cellulose and the most abundant polymeric molecule on earth. It contains about 90% cellulose fibers which is the highest amount among the other sources.
What is Cellulose Journal?
Cellulose Journal is a famous international journal that focuses the research and scientific and technological progress in the field of cellulose and other lignocellulosic polymeric molecule. It publishes the synthesis and characterization of cellulose and other lignocellulosic polymeric molecule with their various applications including energy, fuels, membranes, products with biological, medical, materials, sensors, and biotechnological purposes, and nutrition.
Cellulose Journal Impact Factor
The cellulose Journal Impact Factor is 6.123, which was updated in 2023.
References
Heinze, T. (2016). Cellulose: structure and properties. Cellulose chemistry and properties: fibers, nanocelluloses and advanced materials, 1-52.
Gardner, K. H., & Blackwell, J. (1974). The structure of native cellulose. Biopolymers: Original Research on Biomolecules, 13(10), 1975-2001.
Nishiyama, Y. (2009). Structure and properties of the cellulose microfibril. Journal of wood science, 55(4), 241-249.

