Bases are chemical compounds having hydroxyl ions (OH–) ions and can accept protons in aqueous solutions. Bases have the ability to make red litmus blue and take part in a neutralization reaction with acid molecules to give salt and water. The base compound has a pH value of more than 7. The base has a bitter taste as well as slippery in touch. When a base compound dissolves in water then it is called alkali. Examples: Soap, detergent, toothpaste, metal oxides, metal hydroxides, etc.
Definition of Bases
Bases are chemical compounds that produce OH– ions instead of H+ in the water. When it is soluble in water that known as alkali. The base has the ability to accept a proton from a donor. The weak bases have less ability to accept protons but the strong bases quickly accept protons in solution or from the donor molecules. They have the ability to donate a pair of electrons.
Examples of Bases
There are a lot of bases on our earth for the various fields of applications. Common bases are caustic soda or sodium hydroxide [NaOH], potassium hydroxide [KOH], calcium hydroxide or limewater [Ca(OH)2], magnesium hydroxide [Mg(OH)2], Ammoina (NH3), Lithium hydroxide (LiOH), Acetone (C3H6O), pyridine (C5H5N), Zinc hydroxide [Zn (OH)2], borax, etc. A lot of known substances used in everyday life like soaps, detergents, kinds of toothpaste, and bleaches are the common example of bases.
Theory of Bases
Arrhenius’s Theory of Bases
Bases produce OH– ions in water and increase their concentration when fully dissociating in water. Example: Sodium hydroxide (NaOH) produces OH– in water after dissociation.
NaOH (aq) < —- > Na+ (aq) + OH– (aq)
This theory is unable to explain that the substances lacking hydroxide ions form basic solutions when dissolved in water, such as NO2– and F–.
Bronsted Lowry’s Theory of Bases
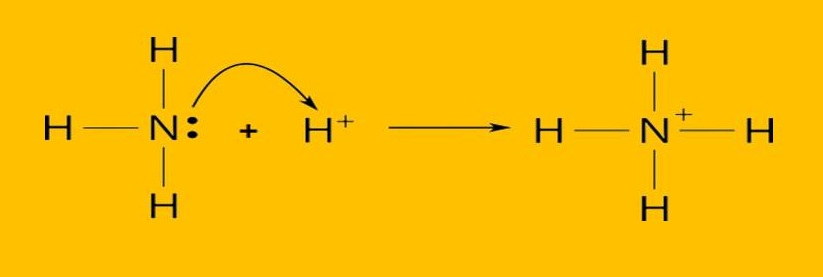
According to this theory, base compounds accept protons from water to produce hydroxide ions. Example: When Ammonia (NH3) reacts with water, it accepts a proton from a water molecule and forms ammonium ion NH4+
NH3 (aq) + H2O (l) < — > NH4+ (aq) + OH– (aq)
Lewis’s Theory of Bases
According to this theory, base compounds are the chemical species that have the ability to donate an electron pair for having a lone pair of electrons in their orbital. Example: Ammonia contains a lone pair of electrons in its orbital which can donate towards the hydrogen ion and able to form Ammonium ion (NH4+)
Classification of Bases
Classification based on their strength properties
The bases are classified into two groups namely strong base and weak base based on their strength.
Strong base: This type of base dissociates completely or almost completely in water and is known as a strong base. Examples: Sodium hydroxide (NaOH), Potassium hydroxide (KOH), Calcium hydroxide [Ca (OH)2], etc.
KOH (aq) <—> K+(aq) + OH–(aq)
Weak base: This type of base dissociates slightly in water and is known as a weak base. Examples: Magnesium hydroxide [Mg (OH)2], Ammonium hydroxide [NH4OH], etc.
NH4OH (aq) <—-> NH4+(aq) + OH–(aq)
Classification based on their concentration properties
They are classified into two categories namely concentrated and dilute base based on their concentration values.
Concentrated Base: The basic solution that contains a higher percentage of the base compound is known as concentrated base. Examples: Concentrated potassium hydroxide (KOH), concentrated ammonium hydroxide (NH4OH), etc.
Diluted Base: The basic solution that contains a relatively small amount of base compound is known as diluted base. Examples: Diluted potassium hydroxide (KOH), dilute ammonium hydroxide (NH4OH), etc
The bases are classified into three categories namely monoacidic base, diacidic base, and triacidic base on the basis of their acidity.
Monoacidic base: This type of base contains only one hydroxyl ion and only combines with one hydrogen ion. Examples: Sodium hydroxide (NaOH), Potassium Hydroxide (KOH), Ammonium hydroxide (NH4OH), etc.
KOH (aq) + HCl (aq) <—> KCl (aq) + H2O (l)
Diacidic base: This type of base contains two hydroxyl ions and only combines with two hydrogen ions.
Examples: Magnesium hydroxide [Mg (OH)2], Ferrous hydroxide [Fe(OH)2], Zinc hydroxide [Zn(OH)2], etc.
Ca (OH)2(aq) + 2HCl(aq) <—> CaCl2(aq) + 2H2O(l)
Triacidic base: This type of base contains three hydroxyl ions and combines with three hydrogen ions. Examples: Aluminium Hydroxide [Al (OH)3], Ferric hydroxide [Fe(OH)3]
Fe (OH)3(aq) + 3HCl(aq) <—> FeCl3(aq) + 3H2O(l)
Properties of Bases
It contains a bitter taste and slippery conditions when touched.
The pH value of base compounds is always greater than 7.
The concentrated basic solution like concentrated sodium hydroxide reacts very quickly with acids such as concentrated sulfuric acid solution.
All basic compounds have the ability to make the red litmus paper blue.
All basic compounds have the ability to impart a yellow color to methyl orange.
All basic compounds have the ability to impart pink color to phenolphthalein.
Basic compounds are good conductors of electric currents in their aqueous state.
Basic compounds are used as electrolytes such as sodium hydroxide (NaOH)
The Basic compounds do not react with metals as acids do.
The basic compounds take part in a neutralization reaction with acid molecules and produce salt and water.
Uses of Bases
Sodium hydroxide (NaOH) base is used in the manufacture of soap, detergent, drain cleaner, and paper. This base is also used in the production of rayon and various types of fabrics.
Calcium hydroxide [Ca(OH)2] or slaked lime or calcium hydroxide is used to make dry mixtures for painting and decorating. It is also used to make cement and lime water.
Magnesium hydroxide or milk of magnesia [Mg (OH)2] is extensively used for making gastric syrup and tablet-making purposes.
Ammonium hydroxide (NH4OH) is used as a critical reagent in industrial sectors.
Lithium Hydroxide (LiOH) is used as a greasing agent.
Barium Hydroxide [Ba(OH)2] is used in laboratories for titration with weak acids.
Sodium bicarbonate (NaHCO3) is used very much for making the baking soda.
Sodium Carbonate (Na2CO3) is used very much for water softening purposes and for making washing soda.
Frequently Asked Questions (FAQs)
What is base in chemistry?
The base is one type of chemical compound that produces hydroxyl ions (OH-) in water. They are generally proton acceptors from acid molecules.
What are the examples of bases?
Sodium hydroxide [NaOH], potassium hydroxide [KOH], calcium hydroxide or limewater [Ca(OH)2], magnesium hydroxide [Mg(OH)2], Ammoina (NH3), Lithium hydroxide (LiOH), Acetone (C3H6O), pyridine (C5H5N), Zinc hydroxide [Zn(OH)2], borax, etc.
What are the types of bases?
The bases are classified into two groups namely strong base and weak base based on their strength. Bases are also classified into two categories namely concentrated and dilute bases based on their concentration values.
What are the uses of bases?
Bases are generally used in fertilizer industries, glass industries, textile industries, paint industries, food industries, paper industries, chemical industries, pharmaceutical industries, etc.
What are the properties of bases?
Bases are bitter in taste and slippery in touch. They show a pH value upper than 7. They participate in the neutralization reaction with acid and form salt with water.
What is a concentrated base?
The basic solution that contains a relatively higher amount of base content is known as a concentrated base.
What is a diluted base?
The basic solution which contains a relatively lower amount of base content is known as diluted base.
What are the examples of diluted bases?
The diluted sodium hydroxide [NaOH], diluted potassium hydroxide [KOH], diluted calcium hydroxide or limewater [Ca(OH)2], diluted magnesium hydroxide [Mg (OH)2], diluted Ammonia (NH3), diluted Lithium hydroxide (LiOH) are the example of diluted base.
What are examples of concentrated bases?
The concentrated solution of sodium hydroxide [NaOH], concentrated solution of potassium hydroxide [KOH], concentrated solution of calcium hydroxide or limewater [Ca(OH)2], concentrated solution of magnesium hydroxide [Mg (OH)2], concentrated solution of Ammoina (NH3), concentrated solution of Lithium hydroxide (LiOH) are the examples of concentrated bases.

