Nuclear reactions are chemical processes used to transform the atomic nuclei into another element by fission or fusion of atomic particles. The atomic nuclei can modified to convert an atom from one element into another through this process. The different types of isotopes of an element or new elements that don’t exist in nature can be generated after nuclear reactions. A lot of stable synthetic elements were created in the late twentieth and early twenty-first centuries by following this reaction.
It is possible to make large atoms in the universe and solar energy in the Sun’s hot through nuclear reactions. The electricity production in nuclear plants as well as the diagnosis of diseases in hospitals can be possible for the nuclear reaction process.
The changes in atomic nuclei in this type of reaction are completely different from the chemical reaction process. The electrons are exchanged between at least two interacting atoms in chemical reactions for producing different chemical compounds but the identity of the atoms remains the same.
On the other hand, the characteristics of neutrons and protons are changed to combine or break down the entire nuclei in nuclear reactions. As a result, the atoms of completely different elements as well as a large amount of energy are produced that can be up to a billion times greater than the conventional chemical reactions.
Nuclear Reactions Definition
It is the chemical process when atomic nuclei or subatomic particles interact with each other to generate different isotopes of an element, or even generate synthetic elements that don’t exist in nature. A large amount of energy is produced after completing this type of reaction.
The representation of this type of reaction is an equation-type style. The participating atomic particle in this reaction is represented as AZX, where X is the symbol of the atomic particle A is the mass number of that atomic particle, and Z is the charge symbol for that particle.
This type of reaction occurs after colliding among the atomic nucleus or subatomic particle to produce one or more new nuclides. A lot of fundamental characteristics or properties of an atomic nucleus are changed in this type of reaction. An unstable radioactive nucleus is generally converted into a stable nucleus without collision in this reaction process. The spontaneous emission of radiation is possible from this process.
History of Nuclear Reactions
Ernest Rutherford conducted of an experiment in 1919 to create an oxygen-17 atom from a nitrogen-14 atom using alpha particles which was the first observation of induced nuclear reactions. The transformation of nitrogen to oxygen is given below:
14N + α → 17O + p.
An important artificial nuclear reaction and nuclear transmutation was successfully done by Rutherford’s colleagues John Cockcroft and Ernest Walton in 1932 at Cambridge University. They used artificially accelerated protons against lithium-7 atoms to split the nucleus into two alpha particles. In 1938, German scientists Otto Hahn, Lise Meitner, and Fritz Strassmann discovered heavy elements by nuclear fission reaction.
Types of Nuclear Reactions and Nuclear Reactions Example
There are two types of nuclear reactions namely nuclear fission and nuclear fusion reaction. The discussions about these types of reactions are given below:
Nuclear Fission Reaction
This type of nuclear reaction relates to the dissociation of an atomic nucleus into two or lighter nuclei. A lot of energy is discharged during the nuclear fission reaction process. The emission of neutrons and gamma rays is generated after completing this reaction. A minimum of two atomic nuclei react with each other to form a single new nucleus. The variation of energy creation from this reaction can be changed over into electricity in the field of nuclear power plants. The generated heat is used to change water into steam

Nuclear Fission Reaction Example
Uranium-235(U-235) atom contains 92 protons and 143 neutrons, for a total of 235. The nucleus of this atom can be disintegrated after being excited by an outside source like absorbing an extra neutron because it is an unstable atom. Then the uranium atom breaks into two parts. Barium, krypton, and three neutrons are then produced. These three neutrons take part in the fission reaction of the other three uranium nuclei to produce 9 neutrons and so on.
Nuclear Fusion Reaction
This type of nuclear reaction relates the association of two atomic nuclei and forms a single new nucleus. A large amount of energy is discharged after completing this reaction. At least two atomic nuclei participate to complete this reaction. The various subatomic particles like neutrons or protons are formed as products after completing this reaction.
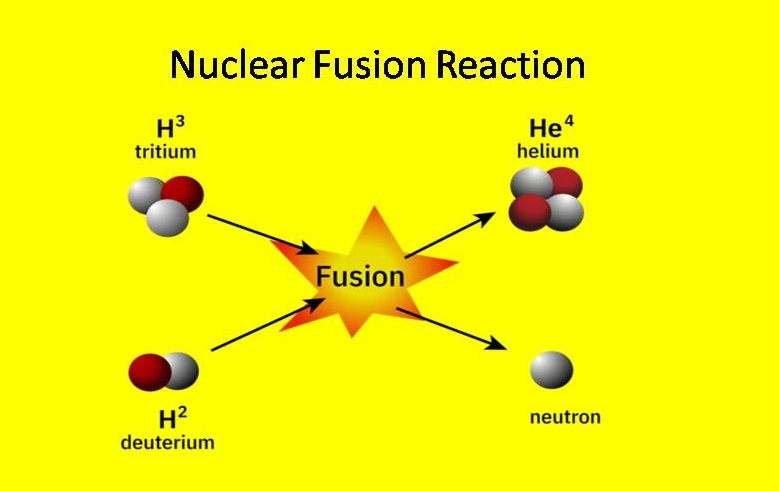
Nuclear Fusion Reaction Example
Deuterium and Tritium isotopes from hydrogen can be combined with each other to form a nucleus of helium and a neutron. A large amount of energy (17.6 MeV) is created after completing this reaction.
Other Types of Nuclear Reactions
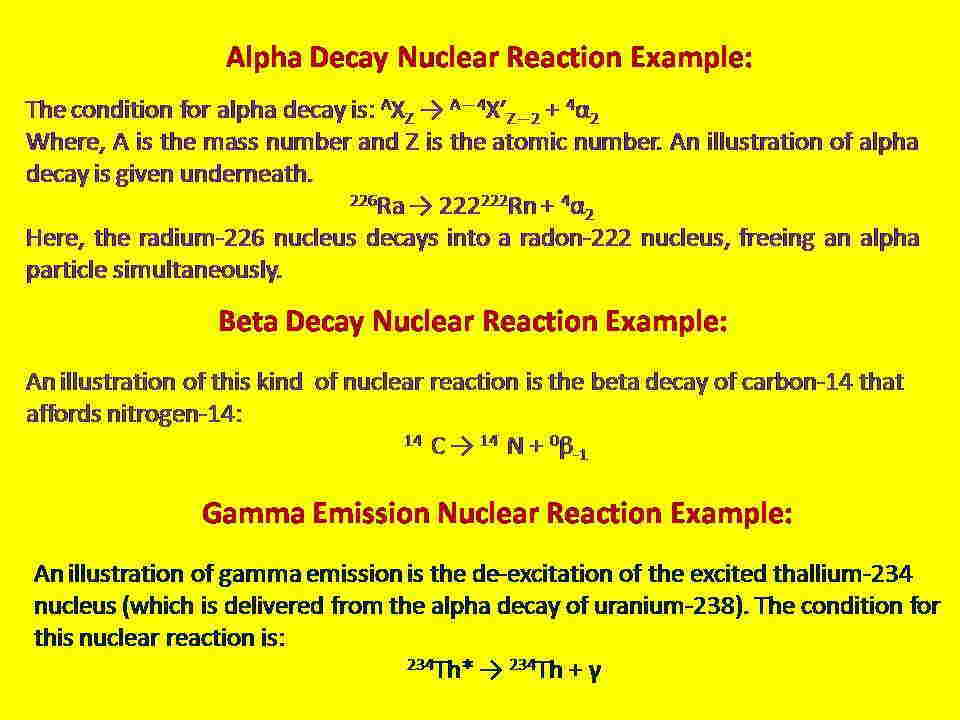
Alpha Decay: This type of nuclear reaction occurs when the nuclei with mass numbers more than 200. This process starts after liberating an alpha particle from the parent nucleus.
Beta Decay: This type of nuclear reaction occurs when a neutron is converted into a proton. This process starts after the emission of a beta particle i.e., a high-energy electron.
Gamma Emission: This reaction starts when an excited nucleus returns to its ground state. This process starts after the emission of a high-energy photon.
Nuclear reactions equation
This equation shows the reactants and products in nuclear fission, nuclear fusion, or other types of radioactive decay. It is written in the style of a chemical reaction equation which contains an initial state and an end state. The scheme of reaction can be written as follows:
Unstable isotope ——————————> More stable isotope + Radiation
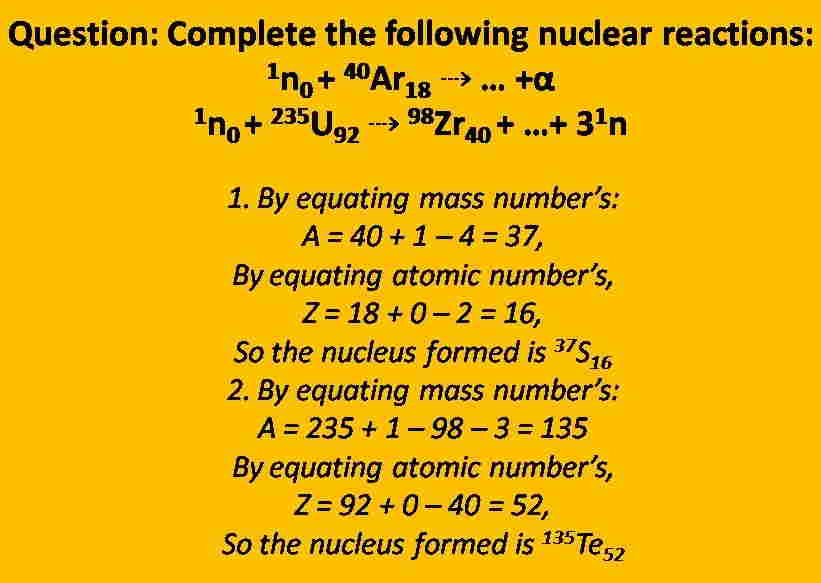
Nuclear Reactions Balancing
A balanced nuclear reaction equation shows that a rearrangement occurs among subatomic particles rather than atoms. There are two ways to balance this type of reaction which is given below:
It must be equal to the sum of the reactant mass numbers to the sum of the mass numbers of the products.
It must be equal to the sum of the reactant’s charge to the sum of the charges of the products.
Image
Nuclear Reactions Energy
A large amount of energy is released after completing nuclear reactions. It occurs when nuclear binding energy is broken. The emission of energy from this reaction occurs by following the equation with respect to the formula-
E=mc2
Where, m= Mass of object, c= velocity of light, and E= Amount of energy liberated during this reaction.
Nuclear Reaction Properties
This type of reaction is irreversible.
It does not follow the law of conservation of mass.
The atomic nuclei transformation takes place.
The reaction characteristics depend on the activity of different types of isotopes.
A lot of energy is created after completing the nuclear reactions.
The mass is not strictly conserved in this type of reaction because some mass is converted into energy by following the equation E = mc2.
Nuclear Reaction and Chemical Reaction Differences
A chemical reaction involves the changes of one or more substances into different substances, whereas a nuclear reaction changes the nucleus of an atom.
A chemical reaction does not release energy, whereas a nuclear reaction produces a large amount of energy.
The chemical reaction does not cause radiation but the nuclear reaction is responsible for creating radiation. The radiation after nuclear reaction is very harmful to people.
Chemical reactions can be reversed but nuclear reactions are irreversible in nature.
Electrons present in orbital take part in chemical reactions but neutrons are the main factors to occur in nuclear reactions.
A chemical reaction occurs after breaking the old chemical bond, whereas a nuclear reaction occurs by combining or breaking the atomic nuclei.
The chemical reaction rate depends on the temperature, pressure concentration, etc. but nuclear reaction rate depends only on nucleons.
Nuclear Reactions Uses
This reaction is used to power spaceships in the extreme environments of deep space which is considered safe, reliable, and maintenance-free for decades of space exploration.
This reaction has great importance in generating electricity which is considered as the most reliable energy source.
This reaction is used in the medical field to diagnose and treat various diseases using radiation or radioactive materials. It helps physicians to identify tumors, size anomalies, or other problems by applying radioisotopes with camera imaging.
It is used to kill cancerous tissue, reduce the size of tumors, and alleviate pain by applying radioisotopes therapeutically.
This reaction is used to get a large amount of energy which can be used to produce high-pressure steam.
Nuclear fusion reaction produces a high amount of energy in the sun, stars, and various planets.
Atomic bombs and hydrogen bombs are produced by following nuclear reactions.
Frequently Asked Questions (FAQs)
What is nuclear reaction?
It is a type of reaction when an atomic nucleus combines with another atomic nucleus or subatomic particle to produce one or more new nuclides. The characteristics of the atomic nucleus are changed after completing a nuclear reaction.
What are the types of nuclear reactions?
There are mainly two types of nuclear reactions namely nuclear fission and nuclear fusion reaction.
What are the examples of nuclear reactions?
21H + 32He —> 24He + 11H
21H + 32He —> 24He + 11H
What are the uses of nuclear reactions?
Nuclear reactions are used in power plants to produce steam and electricity. Nuclear reaction is very important in the field of medicine.
What are the differences between nuclear reactions and chemical reactions?
Nuclear reactions relate to nucleons whereas chemical reactions involve with the transfer of electrons. Nuclear reaction produces a higher amount of energy than chemical reactions. Nuclear reactions are irreversible but chemical reactions can be reversible.
What is nuclear fission reaction?
Nuclear fission reaction involves the bombardment of heavy atomic nuclei with neutrons to produce different types of atomic nuclei with neutrons. Example: 23892U —— > 23490Th + 42He
What is a nuclear fusion reaction?
Nuclear fusion reaction involves the attachment of lighter atomic nuclei at higher temperatures to produce larger atomic nuclei with huge amounts of energy. Example: 21H + 32He —> 24He + 11H

