A pH paper changes it color in various solution and determines basic, acidic or neutral character of the solution. This works done by immersed pH paper into a solution and find out the color change. It changes color in various solutions for the presence of chemical flavin.
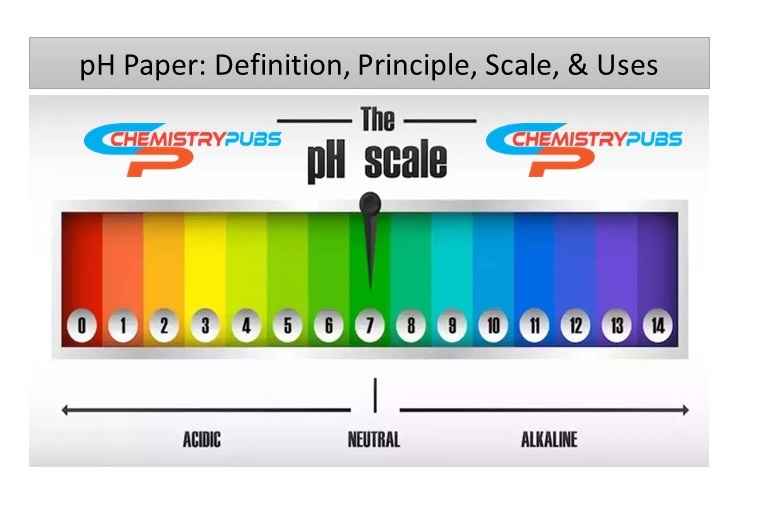
There have many indicators to shows a series of colors from pH 1 to 14. The changes color is then compared with a color chart for determining the pH value. The value of lower pH indicates acid, whereas higher pH indicates base chemical. The value of pH near 7 indicates a neutral chemical substance.
There has various includes a color-coded scale for indicating the pH in this paper.
Introduction about pH
The hydrogen ion concentration in solution to determine the acidity or alkalinity of a solution is known as pH. The scale of pH ranges 0 to 14. Acidic solution shows pH less than 7, whereas basic solution shows pH greater than 7. We can identify the acidic or basic character of solution by determining the pH range. The neutral solution shows the value of pH is 7.
A Dansih biochemist Peter Lauritz was proposed an equation in 1990 for determining the pH value which is given below:
pH = -log[H+]
Where log is the base-10 logarithm and [H+] represents the hydrogen ion concentration in solution. The units of this ion represent the moles per liter solution.
pH paper working principle
pH paper contains Flavin chemical which is soluble in water and shows color changes color in the presence of various types of solutions. This paper becomes red when acidic solution is treated with it. The color of this paper turns into greenish-blue in the presence of alkaline solutions. A neutral solution makes the color of this paper is light green.
A chemical coated indicator in this paper is responsible to form the transition state in the presence of hydrogen or hydroxide ions. Acidic solution forms higher hydrogen ion concentration, whereas basic or alkaline solution forms higher hydroxide ion concentration. Neutral solution contains equal amounts of hydrogen ions and hydroxide ions in the solution.
The coated indicators are generally weak acids or weak bases that change color at specific value of pH. Acid and base chemicals have the ability to exchange a proton. We know, acid donates a proton and base accepts a proton easily. The transfer mechanism of proton into the solution is responsible to changes color of pH paper in the solution.
pH paper or hydrion paper color changes scale
Strong acid shows pH value below 3 which responsible to make red color in this paper. Weak acid has the pH value range 3 to 6 which make orange or yellow color in this paper. Green color comes in this paper when the pH value comes to 7. Weak base contains pH 8 to 11 which shows blue color in this range. pH paper turns into violet or indigo color during the pH range upper than 11.
Precautions to use the pH paper
There have many precautions should be followed during the test with this paper for getting the perfect value which is given below:
It should be used in room temperature because pH varies during changes of temperature.
This paper must be clean.
It should be used very carefully.
It should not used again after using once time.
The color code should be followed carefully during test which provided on the pH paper box.
Advantages of pH Paper
This paper is portable and user-friendly.
This paper can be used for a longer period of time if handled properly.
It is cost-effective and can be used easily.
This paper is available in market area and biodegradable in nature.
Applications of pH paper
This paper is used for knowing the pH of soil sample.
The pH of various biological fluids such as urine, blood, etc. can be determined by helping this paper.
It is heavily used in chemistry laboratory for identifying the acidic, basic or neutral character of the solution.
pH paper is used to know the value of pH of municipal drinking water, swimming pools, and rainwater.
It is used in various chemical industry, pharmaceutical manufacturing, ETP plant, biotechnology, and petroleum based chemical industries.
This paper is very important material to prepare various reagents in microbiology laboratories
This paper is used in food testing laboratory to identify the quality control of food samples for food safety measurement.
Frequently Asked Questions (FAQ’s)
What is pH paper?
pH paper or hydrion paper contains Flavin chemical which is soluble in water and responsible to change color in solution for identifying acidic, basic or neutral character.
What is pH paper used for?
pH paper is used in chemistry laboratory for identifying the acidic, basic or neutral character of the solution.
What are the materials used in pH paper?
A chemical coated indicator in pH creates the transition state in the presence of hydrogen or hydroxide ions and changes color.

