Preparation of standard sodium carbonate solution is a crucial solution for the standardization of secondary standard solution. This solution can be prepared by weighing a definite amount of sodium carbonate chemical and mix with distilled water into a volumetric flask up to the mark for getting a definite volume of standard sodium carbonate solution.
Theory of the preparation of standard sodium carbonate solution
A standard solution is one type of essential solution whose concentration is accurately Known and prepared from a primary standard substance. Anhydrous sodium carbonate is a primary standard substance that is available in a pure state and is water-soluble. It is less hygroscopic in nature and chemically stable. The molar mass or molecular weight of sodium carbonate is 106 g mol-1. Standard sodium carbonate solution can be prepared by weighing a mass of anhydrous sodium carbonate solid and dissolving it in a known volume of solution in a volumetric flask.
Materials required for the preparation of standard sodium carbonate solution
There are many materials required for preparing a standard solution of sodium carbonate that are sodium carbonate, a funnel, distilled water, an electric chemical balance, a watch glass, a weight box, a beaker, a glass rod, a measuring flask, a wash bottle, and a weighing tube.
The calculation for the preparation of standard sodium carbonate solution
Molecular weight of Na2CO3 = {(23×2) + 12 + (3×16)} = 46 + 12 + 48 = 106 gm/mol
Asked concentration, S= 0.03M
The volume of solution=100 ml
We know, W= (SMV/1000)
= {(0.03× 106 × 100)/1000} gm
= 0.318 gm
But, if we take more or less than the above-calculated amount of sodium carbonate, we must calculate again to get the actual concentration of this standard solution.
Suppose, we take 0.359 gm of sodium carbonate instead of the calculated 0.318 gm of sodium carbonate, then we will calculate the by the following method,
Actual concentration= {(Weight taken × Asked concentration)/weight to be taken)}
= {(0.359× 0.03)/0.318}
=0.03387 M
How to prepare a standard sodium carbonate solution?
The procedure for the preparation of standard sodium carbonate solution is given below:
Procedure
At first, take 0.359 gm of Sodium carbonate (Na2CO3) with the help of a weighing scale. Add this chemical into a volumetric flask after placing the funnel on the neck of the volumetric flask. A small amount of distilled water should be added to mix well the sodium carbonate. Then add distilled water carefully up to the mark and make the final volume of 100 ml of this solution. Finally, we get the standard sodium carbonate solution whose concentration is known.
If we use a beaker to prepare standard sodium carbonate solution, mix the sodium carbonate chemical well by using a stirrer with a specific volume of distilled water carefully.
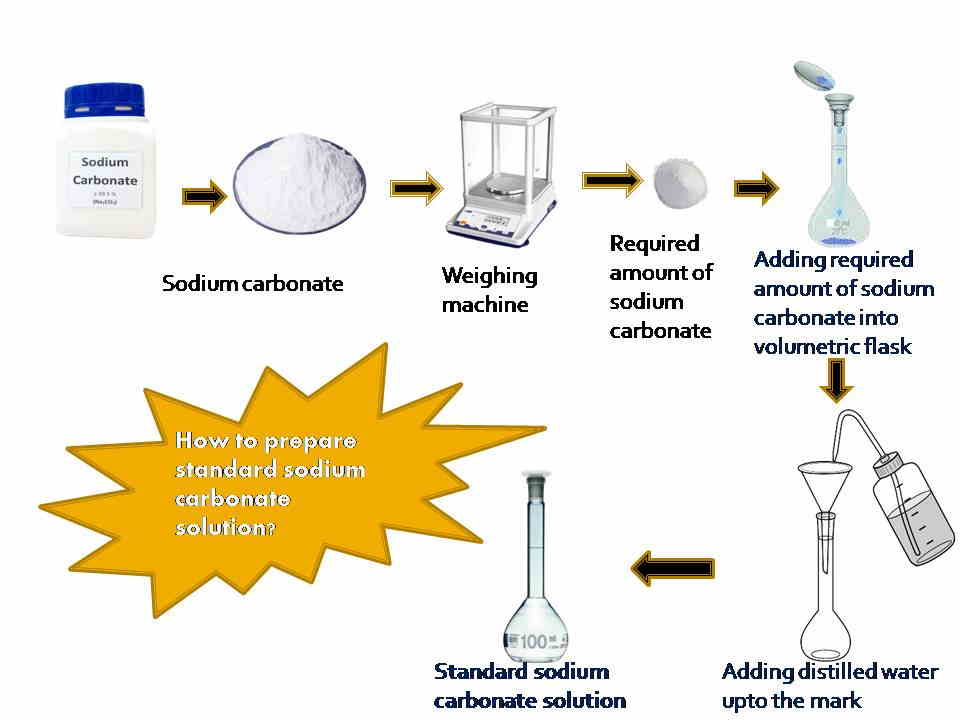
[N.B.= You can prepare different concentrations of standard sodium carbonate solution by following the above method]
Precautions at the time of preparing a standard solution of sodium carbonate
It must avoid any spill of the sodium carbonate on the balance pan.
The apron must be worn at the time of preparing a standard solution of sodium carbonate.
It should handle sodium carbonate carefully because it is corrosive in nature.
The watch glass which is taken must be dry.
The funnel that is taken must be washed thoroughly several times.
It should shake the solution properly.
Result
The strength of the prepared anhydrous sodium carbonate solution is 0.03387 M.
Important question for viva voce
What is a standard solution?
A standard solution is a typical solution in analytical chemistry that contains a definite quantity or substance. A specific amount of solute is dissolved in a solvent to make a standard solution.
How to make a standard solution?
A standard solution preparation relates to the primary standard substance. It is prepared from a primary standard substance. A specific amount of primary standard substance is weighed out by electric balance and then mixed with distilled water to prepare a standard solution.
What is the primary standard substance?
A primary standard substance is a reagent that is very pure, highly stable, nontoxic, inexpensive, less hygroscopic, and has a high molecular weight.
What are the examples of primary standard substances?
Examples of primary standard substances are oxalic acid, sodium carbonate, potassium hydrogen iodate, potassium dichromate, etc.
Is Na2CO3 a primary standard substance?
Sodium carbonate is a common primary standard substance because its solution’s molarity remains constant for a very long period. Sodium carbonate solution is very stable and less hygroscopic in nature.
Washing soda is the common name for?
Washing soda is the common name for Sodium carbonate whose chemical formula is Na2CO3. Washing soda is also known as soda ash.
What is the molecular weight of sodium carbonate?
The molecular weight of sodium carbonate is 106 gm/mol.
What is the formula of sodium carbonate?
The sodium carbonate formula is Na2CO3.
What are the uses of sodium carbonate?
Sodium carbonate is used in chemistry laboratories, textile industries, pharmaceutical industries, cosmetic industries, water treatment industries, glass industries, etc.
What is the molar mass of sodium carbonate?
Sodium carbonate molar mass is 106 gm/mol.
What are the properties of sodium carbonate?
Sodium carbonate properties include odorless, tasteless, highly soluble in water, crystalline solid substance, decomposes at 851°C, and is basic in nature.
What happens when sodium carbonate + hydrochloric acid?
Sodium carbonate + hydrochloric acid produce sodium chloride, carbon dioxide, and water molecules.
Is sodium carbonate acidic or basic?
The sodium carbonate formula is Na2CO3, so it is basic in nature.
What is the pH of sodium carbonate solution?
The pH of sodium carbonate solution ranges between 10 to 11.5.
What is the sodium carbonate Sanket?
Sodium carbonate Sanket is Na2CO3.
What is the formula of washing soda?
The washing soda formula is Na2CO3·10H2O.
What is the chemical name of washing soda?
Washing soda’s chemical name is sodium carbonate decahydrate.
What are the properties of Sodium carbonate decahydrate?
Sodium carbonate decahydrate is a water-insoluble chemical and can be converted to other Sodium compounds. The sodium carbonate decahydrate melting point is 34 °C. Sodium carbonate decahydrate boiling point is 333.6 °C. Sodium carbonate decahydrate molecular weight is 286.14.
What are the uses of washing soda?
Washing soda is used as a laboratory reagent and as a raw material for the manufacture of soap, paper, and glass. Washing soda is also used in textiles and petroleum refining purposes.
What is the chemical formula of sodium carbonate decahydrate?
The sodium carbonate decahydrate chemical formula is Na2CO3.10H2O.

