Standardization of NaOH with Oxalic Acid relates to the neutralization reaction. It is also called acidimetry because the process of standardization of Sodium hydroxide with Oxalic Acid reveals the strength of the Alkali solution.
Aim of the standardization of Sodium hydroxide with Oxalic Acid
This process completely relates the evolution of a given solution of sodium hydroxide by titrating it against a standard oxalic acid solution.
Theory of Standardization of NaOH with Oxalic Acid
Sodium hydroxide is a secondary standard and oxalic acid is a primary standard. So the supplied NaOH can be standardized by the standard oxalic acid solution.
Reaction: 2NaOH + (COOH)2.2H2O =2COONa + 4H2O
So, it can be said that 2-mole sodium hydroxide reacts with 1-mole oxalic acid.
2 mole NaOH =1 mole (COOH)2.2H2O
Apparatus for Standardization of NaOH with Oxalic Acid
Conical flask, Burette, Pipette, Funnel, Volumetric flask, Electric balance, Beaker
Reagents and Chemicals for Standardization of NaOH with Oxalic Acid
Sodium hydroxide NaOH and Oxalic acid (COOH)2.2H2O
Indicator for Standardization of NaOH with Oxalic Acid
Phenolphthalein indicator.
Procedure for Standardization of NaOH with Oxalic Acid
Step-1: Preparation of 0.1 (M) oxalic acid solution
Make 100 mL of this solution by dissolving the calculated amount of oxalic acid dihydrate in 100 mL of distilled water in a 100 mL volumetric flask. Use electronic balance very carefully to measure the amount of oxalic acid dehydrated.
Step 2: Standardization of the supplied sodium hydroxide solution
Fill a clean and dry burette with the supplied sodium hydroxide fixed to a stand with a clamp. Adjust the volume to a convenient known value by tapping out all the air bubbles at the bottom of the burette and note this in your lab book.
Take 10 ml of the prepared 0.1M oxalic acid solution in a 250 ml conical flask.
Add 1 drop of phenolphthalein indicator and note the color of the solution.
Now, titrate by adding acid dropwise from the burette into the solution of the conical flask. This work must continue until the phenolphthalein becomes light pink and then wash the conical flask by spraying a little distilled water from the wash bottle.
Continue titration very carefully until the color becomes faint pink and note in your lab book.
Find the volume of consumed acid from initial and final readings.
Perform two more titrations calculate the volume of consumed sodium hydroxide and note them in your lab book.
Take the average of these three volumes.
Now, calculate the strength of the supplied sodium hydroxide.
Observations for the Titration of NaOH with Oxalic Acid
The strength or Molarity of sodium hydroxide solution = x
The Volume of taken oxalic acid solution = 10ml
Indicator for this experiment = Phenolphthalein
The endpoint of this experiment is = Light pink color
Table for the Standardization of NaOH with Oxalic Acid
The table for the Standardization of sodium hydroxide with Standard oxalic acid solution is shown below: (N.B.: All of the following values can be changed during your experiments based on requirements)
| Obs. No. | The average volume of NaOH consumed (mL) | Initial Burette reading (mL) | Final Burette reading (mL) | Amount of NaOH consumed (mL) | Average volume of NaOH consumed (mL) |
| 1. | 10 | 40 | 26.9 | 13.1 | |
| 2. | 10 | 26.9 | 13.6 | 13.3 | 13.1666 |
| 3. | 10 | 13.6 | 0.5 | 13.1 |
Calculation for the Standardization of Sodium hydroxide with Oxalic Acid
From the balanced neutralization reaction equation,
We get the following:
2 mole NaOH =1 mole (COOH)2.2H2O
We apply the following formula,
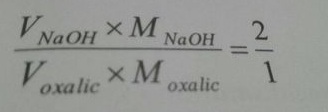
Or, , MNaOH= { (2 x V0xalicacid x MOxalic Acid ) / VNaOH
={ (2 x10 x 0.1) /12.833} = 0.1558 M (Ans.)
Result: The concentration of sodium hydroxide (NaOH) is 0.1558 M.
Precautions for the Titration of NaOH with Oxalic Acid
It should weigh the oxalic acid crystals very carefully.
It must avoid spilling the substance on the balance pan at the time of weighing.
It should wash the burette with water before and after the titration.
It should carefully addition of distilled water up to the mark on the neck of the measuring cylinder and avoid extra addition of distilled water.
Important Questions for Viva Voce
What is a standard solution in chemistry?
A standard solution is one type of chemical solution whose concentration is accurately known.
How to prepare a standard solution?
Preparation of a standard solution relates to the dissolving of a known mass of solute and dilutes the solution to make a definite volume.
What are the examples of standard solutions?
Examples of a standard solution: are 0.1 M oxalic acid solution, 0.1 M Sodium Carbonate solution, etc.
What are the types of Standard solutions?
Types of standard solutions are Primary standard solutions and Secondary standard solutions.
What is the primary standard solution?
A primary standard solution is an ultrapure compound that acts as the reference material for titration.
What are the examples of primary standard solutions?
Examples of primary standard solutions are 0.1 M Oxalic acid solution and 0.1 M Sodium Carbonate solution.
What is a secondary standard solution?
A secondary standard solution is a solution whose concentration can be determined by titration with a primary standard solution.
What are the examples of secondary standard solutions?
Examples of secondary standard solutions are Sodium hydroxide, Potassium hydroxide, Sulphuric acid, Nitric acid, etc.
What is Molar Solution in Chemistry?
The molar solution defines the gram molecular weight of a solute per liter (L) of solution.
What is a molar solution formula?
The molar solution formula is given by,
S= 1000w/MV where, S= Molarity, w= weight of solute, M= Molecular weight of solute, V=volume of solution.
What is a molar solution example?
The molar solution examples are 0.1M Oxalic acid solution, 0.1 M Sodium Carbonate solution, etc.
What is acidimetry?
Acidimetry relates to the determination of the strength of an acid by titrating it against a standard alkali solution by using a suitable indicator.
What type of reaction is an acid-alkali titration?
An acid-alkali titration relates to the Neutralization titration.
What is an indicator in chemistry?
The indicator is a weak acid or a weak base that changes in color for the changes in concentration of Hydrogen ions in a solution or the pH of a solution changes. Indicators are slightly dissolved in the water to form ions.
What are the examples of indicators in chemistry?
Examples of indicators are Litmus, Methyl orange, Phenolphthalein, etc.
What are the differences between primary standard substances and secondary standard substances?
Difference between primary standard substances and secondary standard substances on the basis of definition
The primary standard substance is the purest compound and acts as the reference material for quantitative analysis.
A secondary standard substance is a compound whose concentration can be determined by chemical analysis.
Difference between primary standard substances and secondary standard substances on the basis of purity
A primary standard substance is regarded as a very pure compound.
A secondary standard substance is regarded as a less pure compound than a primary standard substance.
Difference between primary standard substances and secondary standard substances on the basis of reactivity
The primary standard substance shows less reactivity.
The secondary standard substance shows high reactivity.
Difference between primary standard substances and secondary standard substances on the basis of availability
The primary standard substance is available in the market.
The secondary standard substance is not available in the market.
Difference between primary standard substances and secondary standard substances on the basis of stability
Primary standard substance is more stable than secondary standard substance.
Secondary standard substance is less stable than primary standard substance.
What are the types of indicators?
There are two types of Indicators namely natural indicators and artificial indicators.
What is standardization in Chemistry?
Standardization in Chemistry relates to the determination of the definite concentration (molarity) of a solution.
What is titration?
Titration is a method to determine the concentration of an unknown solution by reacting it with a solution whose concentration is precisely known.
What is titrant?
Titrant is a solution whose concentration is definitely known. The titrant is also known as the titrator, the reagent, or the standard solution.
What is titrand?
Titrand or analyte is the substance whose concentration is being determined by reacting it with the titrant. The concentration of Titrand or analyte solution is unknown.
What is the neutralization reaction?
Neutralization is a type of chemical reaction related to the reaction between an acid and a base to form salt and water. The pH of a solution of this reaction completely depends upon the acid strength of the reactants and their concentrations.
What is molecular weight?
Molecular weight is commonly abbreviated by M.W. or MW which is commonly used in chemistry to measure the sum of the atomic weight values of the atoms in a molecule.
What is the formula of sodium hydroxide?
The sodium hydroxide formula is NaOH.
What is the molecular weight of sodium hydroxide?
The molecular weight of sodium hydroxide is 40.
What are the properties of Sodium hydroxide?
Sodium hydroxide or caustic soda is a common inorganic and white solid ionic compound compound with the formula NaOH. It consists of sodium cations Na+ and hydroxide anions OH−. Sodium hydroxide or caustic soda is very soluble in water, and quickly absorbs moisture and carbon dioxide from the air.
What is sodium hydroxide used for?
Sodium hydroxide is used in the production of pulp and paper from wood, textiles, safe drinking water, high-quality soaps, and detergents, and as a drain opener.
Is NaOH a base or acid?
Sodium Hydroxide or NaOH is a base that contains hydroxide-ion and releases it in water.
What is the formula of oxalic acid?
Oxalic acid formula is C2H2O4. It has a condensed formula HOOC-COOH.
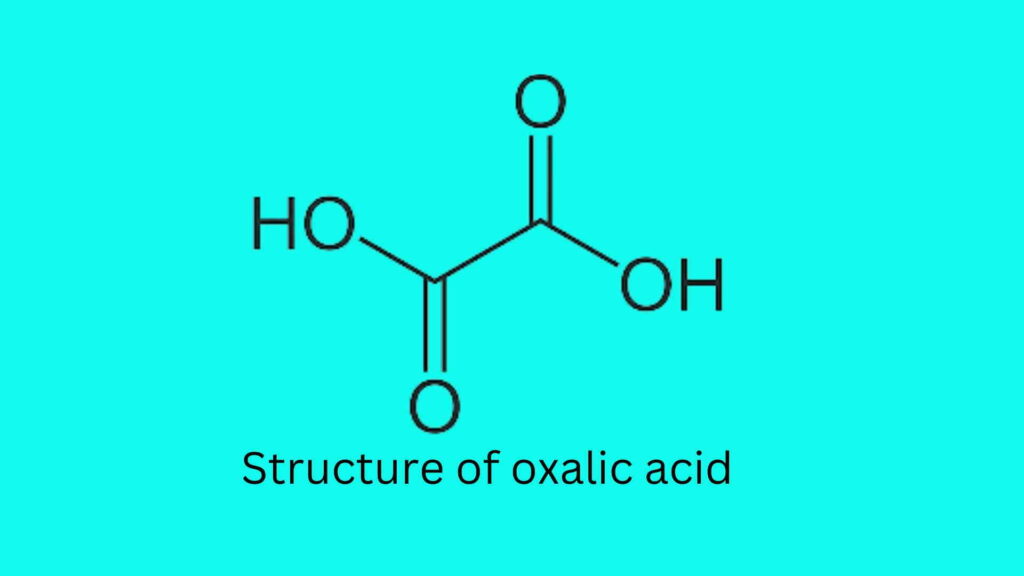
What is the structure of oxalic acid?
Oxalic acid formula is C2H2O4 which is the crystalline hydrate, (COOH)2·2H2O. The structure of oxalic is given below:
What is the molecular weight of oxalic acid?
Oxalic acid formula is C2H2O4. So, the molecular weight of oxalic acid is 90g/mol.
Is oxalic acid molar mass 126 or 90?
Oxalic acid is mostly found in crystalline hydrate whose formula is H2C2O4.2H2O. After calculation, we get the molecular mass of the oxalic acid is 126 g/mol.
What is the symbol of oxalic acid?
The oxalic acid symbol is H2C2O4.
What is the IUPAC name of oxalic acid?
Oxalic acid IUPAC name is Ethanedioic acid which is a colorless, crystalline, and toxic organic compound.
What is the equivalent weight of oxalic acid?
The equivalent weight of oxalic acid is 63 which is obtained after dividing the molecular weight of this acid by n-factor (valency=2).
What is Basicity?
The basicity of an acid molecule relates to the ability to produce hydrogen ( H +) ions in an aqueous solution.
What is the basicity of oxalic acid crystals?
Oxalic acid’s basicity is 2 because it is a dibasic acid that contains two protons that can be replaced.
What happens when NaOH reacts with oxalic acid?
Oxalic acid+ NaOH produces sodium carboxylate and water molecules.
What is the balanced equation for oxalic acid with sodium hydroxide?
Oxalic acid sodium hydroxide hydroxide balanced equation is
2NaOH + (COOH)2.2H2O =2COONa + 4H2O

